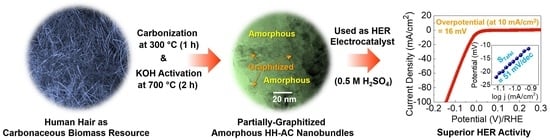
Excellent Electrocatalytic Hydrogen Evolution Reaction Performances of Partially Graphitized Activated-Carbon Nanobundles Derived from Biomass Human Hair Wastes
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of HH-AC Nanobundles
2.2. Material Characterizations
2.3. Electrocatalytic Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Liu, Y.; Wu, M.; Feng, X.; Redfern, S.A.T.; Shang, Y.; Yong, X.; Feng, T.; Wu, K.; Liu, Z.; et al. Carbon-Quantum-Dots-Loaded Ruthenium Nanoparticles as an Efficient Electrocatalyst for Hydrogen Production in Alkaline Media. Adv. Mater. 2018, 30, 1800676. [Google Scholar] [CrossRef] [PubMed]
- Sapountzi, F.M.; Gracia, J.M.; Weststrate, C.J.; Fredriksson, H.O.A.; Niemantsverdriet, J.W. Electrocatalysts for the generation of hydrogen, oxygen and synthesis gas. Prog. Energy Combust. Sci. 2017, 58, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Guan, J.; Tu, Y.; Chen, S.; Wang, Y.; Wang, S.; Yu, L.; Ma, C.; Deng, D.; Bao, X. Highly efficient H2 production from H2S via a robust graphene-encapsulated metal catalyst. Energy Environ. Sci. 2020, 13, 119–126. [Google Scholar] [CrossRef]
- Jin, H.; Guo, C.; Liu, X.; Liu, J.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S.-Z. Emerging Two-Dimensional Nanomaterials for Electrocatalysis. Chem. Rev. 2018, 118, 6337–6408. [Google Scholar] [CrossRef]
- Sekar, S.; Aqueel Ahmed, A.T.; Pawar, S.M.; Lee, Y.; Im, H.; Kim, D.Y.; Lee, S. Enhanced water splitting performance of biomass activated carbon-anchored WO3 nanoflakes. Appl. Surf. Sci. 2020, 508, 145127. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhong, C.-J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef]
- Xu, S.; Gong, S.; Jiang, H.; Shi, P.; Fan, J.; Xu, Q.; Min, Y. Z-scheme heterojunction through interface engineering for broad spectrum photocatalytic water splitting. Appl. Catal. B Environ. 2020, 267, 118661. [Google Scholar] [CrossRef]
- Jaleh, B.; Nasrollahzadeh, M.; Nasri, A.; Eslamipanah, M.; Moradi, A.; Nezafat, Z. Biopolymer-derived (nano)catalysts for hydrogen evolution via hydrolysis of hydrides and electrochemical and photocatalytic techniques: A review. Int. J. Biol. Macromol. 2021, 182, 1056–1090. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, R.; Wang, Z.; Shen, D.; Yu, R.; Luo, K.; Wu, C.; Gu, S. Progress in carbon-based electrocatalyst derived from biomass for the hydrogen evolution reaction. Fuel 2021, 293, 120440. [Google Scholar] [CrossRef]
- Ghosh, S.; Basu, R.N. Multifunctional nanostructured electrocatalysts for energy conversion and storage: Current status and perspectives. Nanoscale 2018, 10, 11241–11280. [Google Scholar] [CrossRef]
- Ito, Y.; Cong, W.; Fujita, T.; Tang, Z.; Chen, M. High Catalytic Activity of Nitrogen and Sulfur Co-Doped Nanoporous Graphene in the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2015, 54, 2131–2136. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, Z.; Wang, Y.; Cao, E.; Xiao, F.; Chen, S.; Du, S.; Wu, Y.; Ren, Z. Regulating the allocation of N and P in codoped graphene via supramolecular control to remarkably boost hydrogen evolution. Energy Environ. Sci. 2019, 12, 2697–2705. [Google Scholar] [CrossRef]
- Yan, X.; Jia, Y.; Odedairo, T.; Zhao, X.; Jin, Z.; Zhu, Z.; Yao, X. Activated carbon becomes active for oxygen reduction and hydrogen evolution reactions. Chem. Commun. 2016, 52, 8156–8159. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, K.R.A.; Prabu, N.; Sasidharan, M.; Maduraiveeran, G. Nitrogen-self doped activated carbon nanosheets derived from peanut shells for enhanced hydrogen evolution reaction. Appl. Surf. Sci. 2019, 489, 725–733. [Google Scholar] [CrossRef]
- Prabu, N.; Kesavan, T.; Maduraiveeran, G.; Sasidharan, M. Bio-derived nanoporous activated carbon sheets as electrocatalyst for enhanced electrochemical water splitting. Int. J. Hydrog. Energy 2019, 44, 19995–20006. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; Ren, T.-Z.; Yuan, Z.-Y.; Bandosz, T.J. Activated carbon with heteroatoms from organic salt for hydrogen evolution reaction. Micropor. Mesopor. Mat. 2020, 297, 110033. [Google Scholar] [CrossRef]
- Akyüz, D.; Keskin, B.; Şahintürk, U.; Koca, A. Electrocatalytic hydrogen evolution reaction on reduced graphene oxide electrode decorated with cobaltphthalocyanine. Appl. Catal. B 2016, 188, 217–226. [Google Scholar] [CrossRef]
- Qu, K.; Zheng, Y.; Jiao, Y.; Zhang, X.; Dai, S.; Qiao, S.-Z. Polydopamine-Inspired, Dual Heteroatom-Doped Carbon Nanotubes for Highly Efficient Overall Water Splitting. Adv. Energy Mater. 2017, 7, 1602068. [Google Scholar] [CrossRef] [Green Version]
- Elizabeth, I.; Nair, A.K.; Singh, B.P.; Gopukumar, S. Multifunctional Ni-NiO-CNT Composite as High Performing Free Standing Anode for Li Ion Batteries and Advanced Electro Catalyst for Oxygen Evolution Reaction. Electrochim. Acta 2017, 230, 98–105. [Google Scholar] [CrossRef]
- Cui, W.; Liu, Q.; Cheng, N.; Asiri, A.M.; Sun, X. Activated carbon nanotubes: A highly-active metal-free electrocatalyst for hydrogen evolution reaction. Chem. Commun. 2014, 50, 9340–9342. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Meng, S.; Ye, X.; Fu, X.; Chen, S. Controlled synthesis of Sn-based oxides via a hydrothermal method and their visible light photocatalytic performances. RSC Adv. 2017, 7, 27024–27032. [Google Scholar] [CrossRef] [Green Version]
- Shinde, S.S.; Sami, A.; Lee, J.-H. Electrocatalytic hydrogen evolution using graphitic carbon nitride coupled with nanoporous graphene co-doped by S and Se. J. Mater. Chem. A 2015, 3, 12810–12819. [Google Scholar] [CrossRef]
- Sathiskumar, C.; Ramakrishnan, S.; Vinothkannan, M.; Rhan Kim, A.; Karthikeyan, S.; Yoo, D.J. Nitrogen-Doped Porous Carbon Derived from Biomass Used as Trifunctional Electrocatalyst toward Oxygen Reduction, Oxygen Evolution and Hydrogen Evolution Reactions. Nanomaterials 2020, 10, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Zhou, W.; Yang, L.; Li, L.; Zhang, Z.; Ke, Y.; Chen, S. Nitrogen and sulfur co-doped porous carbon derived from human hair as highly efficient metal-free electrocatalysts for hydrogen evolution reactions. J. Mater. Chem. A 2015, 3, 8840–8846. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, M.; Xu, W.; Wu, X.; Jiang, J. Catalytically Active Carbon from Cattail Fibers for Electrochemical Reduction Reaction. Front. Chem. 2019, 7, 786. [Google Scholar] [CrossRef]
- Wang, J.; Kong, H.; Zhang, J.; Hao, Y.; Shao, Z.; Ciucci, F. Carbon-based electrocatalysts for sustainable energy applications. Prog. Mater. Sci. 2021, 116, 100717. [Google Scholar] [CrossRef]
- Sekar, S.; Kim, D.Y.; Lee, S. Excellent Oxygen Evolution Reaction of Activated Carbon-Anchored NiO Nanotablets Prepared by Green Routes. Nanomaterials 2020, 10, 1382. [Google Scholar] [CrossRef]
- Atif, M.; Farid, M.Q.; Ahmad, S.A.; Abdul Karim, R.; Hussain, A.; Rabbani, F.; Bongiovanni, R. Electrochemical Evaluation of Human Hair Derived Carbon Particles. ECS J. Solid State Sci. Technol. 2020, 9, 051003. [Google Scholar] [CrossRef]
- Sankar, S.; Ahmed, A.T.A.; Inamdar, A.I.; Im, H.; Im, Y.B.; Lee, Y.; Kim, D.Y.; Lee, S. Biomass-derived ultrathin mesoporous graphitic carbon nanoflakes as stable electrode material for high-performance supercapacitors. Mater. Des. 2019, 169, 107688. [Google Scholar] [CrossRef]
- Manasa, P.; Lei, Z.J.; Ran, F. Biomass Waste Derived Low Cost Activated Carbon from Carchorus Olitorius (Jute Fiber) as Sustainable and Novel Electrode Material. J. Energy Storage 2020, 30, 101494. [Google Scholar] [CrossRef]
- Sekar, S.; Aqueel Ahmed, A.T.; Inamdar, A.I.; Lee, Y.; Im, H.; Kim, D.Y.; Lee, S. Activated carbon-decorated spherical silicon nanocrystal composites synchronously-derived from rice husks for anodic source of lithium-ion battery. Nanomaterials 2019, 9, 1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, W.; Sun, F.; Xu, Y.; Qiu, L.; Liu, C.; Wang, S.; Yan, F. Human hair-derived carbon flakes for electrochemical supercapacitors. Energy Environ. Sci. 2014, 7, 379–386. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-Based Supercapacitors Produced by Activation of Graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekar, S.; Aqueel Ahmed, A.T.; Kim, D.Y.; Lee, S. One-Pot Synthesized Biomass C-Si Nanocomposites as an Anodic Material for High-Performance Sodium-Ion Battery. Nanomaterials 2020, 10, 1728. [Google Scholar] [CrossRef]
- Sekar, S.; Lee, Y.; Kim, D.Y.; Lee, S. Substantial LIB anode performance of graphitic carbon nanoflakes derived from biomass green-tea waste. Nanomaterials 2019, 9, 871. [Google Scholar] [CrossRef] [Green Version]
- Sankar, S.; Lee, H.; Jung, H.; Kim, A.; Aqueel Ahmed, A.T.; Inamdar, A.I.; Kim, H.; Lee, S.; Im, H.; Kim, D.Y. Ultrathin graphene nanosheets derived from rice husks for sustainable supercapacitor electrodes. New J. Chem. 2017, 41, 13792–13797. [Google Scholar] [CrossRef]
- Sekar, S.; Lee, S.; Vijayarengan, P.; Kalirajan, K.M.; Santhakumar, T.; Sekar, S.; Sadhasivam, S. Upcycling of Wastewater via Effective Photocatalytic Hydrogen Production Using MnO2 Nanoparticles—Decorated Activated Carbon Nanoflakes. Nanomaterials 2020, 10, 1610. [Google Scholar] [CrossRef]
- Sekar, S.; Lee, S.; Kim, D.Y.; Preethi, V.; Kalirajan, K.M.; Sutha, S.; Saravanan, S.; Therli, A.; Roy, M.; Jagannathan, K. Biomass activated carbon-decorated spherical β-Ni(OH)2 nanoparticles for enhanced hydrogen production from sulphide wastewater. J. Water Process. Eng. 2020, 38, 101669. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Islam, M.A.; Asif, M.; Hameed, B.H. Human hair-derived high surface area porous carbon material for the adsorption isotherm and kinetics of tetracycline antibiotics. Bioresour. Technol. 2017, 243, 778–784. [Google Scholar] [CrossRef]
- Rajagopal, R.R.; Aravinda, L.S.; Rajarao, R.; Bhat, B.R.; Sahajwalla, V. Activated carbon derived from non-metallic printed circuit board waste for supercapacitor application. Electrochim. Acta 2016, 211, 488–498. [Google Scholar] [CrossRef]
- Eleri, O.E.; Azuatalam, K.U.; Minde, M.W.; Trindade, A.M.; Muthuswamy, N.; Lou, F.; Yu, Z. Towards high-energy-density supercapacitors via less-defects activated carbon from sawdust. Electrochim. Acta 2020, 362, 137152. [Google Scholar] [CrossRef]
- Yu, A.; Kim, S.Y.; Lee, C.; Kim, M.H.; Lee, Y. Boosted Electron-Transfer Kinetics of Hydrogen Evolution Reaction at Bimetallic RhCo Alloy Nanotubes in Acidic Solution. ACS Appl. Mater. Interfaces 2019, 11, 46886–46893. [Google Scholar] [CrossRef] [PubMed]
- Aqueel Ahmed, A.T.; Pawar, S.M.; Inamdar, A.I.; Kim, H.; Im, H. A Morphologically Engineered Robust Bifunctional CuCo2O4 Nanosheet Catalyst for Highly Efficient Overall Water Splitting. Adv. Mater. Interfaces 2020, 7, 1901515. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.T.A.; Pawar, S.M.; Inamdar, A.I.; Im, H.; Kim, H. Fabrication of FeO@CuCo2S4 multifunctional electrode for ultrahigh-capacity supercapacitors and efficient oxygen evolution reaction. Int. J. Energy Res. 2020, 44, 1798–1811. [Google Scholar] [CrossRef]
- Schonvogel, D.; Nowotny, M.; Woriescheck, T.; Multhaupt, H.; Wagner, P.; Dyck, A.; Agert, C.; Wark, M. Hydrothermal Carbonization-Derived Carbon from Waste Biomass as Renewable Pt Support for Fuel Cell Applications: Role of Carbon Activation. Energy Technol. 2019, 7, 1900344. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Pan, Y.; Sorrell, C.C.; Du, H. Assessment of electrocatalytic activity through the lens of three surface area normalization techniques. J. Mater. Chem. A 2020, 8, 3154–3159. [Google Scholar] [CrossRef]
- Ullah, N.; Zhao, W.; Lu, X.; Oluigbo, C.J.; Shah, S.A.; Zhang, M.; Xie, J.; Xu, Y. In situ growth of M-MO (M = Ni, Co) in 3D graphene as a competent bifunctional electrocatalyst for OER and HER. Electrochim. Acta 2019, 298, 163–171. [Google Scholar] [CrossRef]
- Hoang, V.C.; Dinh, K.N.; Gomes, V.G. Hybrid Ni/NiO composite with N-doped activated carbon from waste cauliflower leaves: A sustainable bifunctional electrocatalyst for efficient water splitting. Carbon 2020, 157, 515–524. [Google Scholar] [CrossRef]
- Qu, K.; Zheng, Y.; Zhang, X.; Davey, K.; Dai, S.; Qiao, S.Z. Promotion of Electrocatalytic Hydrogen Evolution Reaction on Nitrogen-Doped Carbon Nanosheets with Secondary Heteroatoms. ACS Nano 2017, 11, 7293–7300. [Google Scholar] [CrossRef]







| Catalyst | Overpotential η (mV) | Tafel Slope ST (mV/dec) | Electrolytes | Reference |
|---|---|---|---|---|
| HH-AC-700 layered nanobundles | 16 | 51 | 0.5 M H2SO4 | This Work |
| HH-AC-600 nanobundles | 34 | 72 | 0.5 M H2SO4 | This Work |
| N- and S-codoped graphene | 276 | 81 | 0.5 M H2SO4 | [11] |
| N- and P-codoped graphene | 106 | 67.3 | 0.5 M H2SO4 | [12] |
| Defective AC | 334 | 66 | 0.5 M H2SO4 | [13] |
| N-doped AC | 80 | 75 | 0.5 M H2SO4 | [14] |
| Nanoporous AC | 380 | 85 | 0.5 M H2SO4 | [15] |
| N- and S-codoped AC | 450 | 163 | 1 M KOH | [16] |
| N- and S-codoped CNT * | 450 | 133 | 1 M KOH | [18] |
| Ni-NiO-CNT composite | 276 | 94 | 1 M H2SO4 | [19] |
| Activated CNT * | 225 | 71 | 0.5 M H2SO4 | [20] |
| N-doped porous carbon | 179 | 98 | 1 M KOH | [23] |
| N- and S-codoped porous carbon | 12 | 57.4 | 0.5 M H2SO4 | [24] |
| N-doped carbon fiber | 150 | 89 | 0.5 M H2SO4 | [25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekar, S.; Sim, D.H.; Lee, S. Excellent Electrocatalytic Hydrogen Evolution Reaction Performances of Partially Graphitized Activated-Carbon Nanobundles Derived from Biomass Human Hair Wastes. Nanomaterials 2022, 12, 531. https://doi.org/10.3390/nano12030531
Sekar S, Sim DH, Lee S. Excellent Electrocatalytic Hydrogen Evolution Reaction Performances of Partially Graphitized Activated-Carbon Nanobundles Derived from Biomass Human Hair Wastes. Nanomaterials. 2022; 12(3):531. https://doi.org/10.3390/nano12030531
Chicago/Turabian StyleSekar, Sankar, Dae Hyun Sim, and Sejoon Lee. 2022. "Excellent Electrocatalytic Hydrogen Evolution Reaction Performances of Partially Graphitized Activated-Carbon Nanobundles Derived from Biomass Human Hair Wastes" Nanomaterials 12, no. 3: 531. https://doi.org/10.3390/nano12030531