Cascade Förster Resonance Energy Transfer Studies for Enhancement of Light Harvesting on Dye-Sensitized Solar Cells
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials and Instruments
2.2. DSSC Measurements
3. Results and Discussion
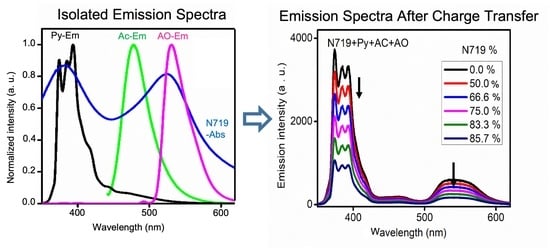
3.1. Optimization of Cascade FRET
3.2. Energy Transfer in Cascade FRET
3.3. Enhancement of Light Harvesting on DSSC Systems Equipped Cascade FRET
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gregg, B.A.; Sprague, J.; Peterson, M.W. Long-Range Singlet Energy Transfer in Perylene Bis(phenethylimide) Films. J. Phys. Chem. B 1997, 101, 5362–5369. [Google Scholar] [CrossRef]
- Howe, B.; Diaz, A.L. Characterization of host-lattice emission and energy transfer in BaMgAl10O17: Eu2+. J. Lumin. 2004, 109, 51–59. [Google Scholar] [CrossRef]
- Fujii, K.; Kuroda, T.; Sakoda, K.; Iyi, N. Fluorescence resonance energy transfer and arrangements of fluorophores in integrated coumarin/cyanine systems within solid-state two-dimensional nanospace. J. Photochem. Photobiol. A Chem. 2011, 225, 125–134. [Google Scholar] [CrossRef]
- Valeur, B. Effects of Intermolecular Photophysical Processes on Fluorescence Emission in Molecular Fluorescence; Wiley: Weinheim, Germany, 2001; pp. 72–124. [Google Scholar]
- Cheon, J.H.; Jung, D.Y.; Choi, S.K.; Ahn, K.S.; Lee, D.K.; Kim, J.H. Enhancement of Light Harvesting in Dye-Sensitized Solar Cells by using Först-Type Resonance Energy Transfer. Met. Mater. Int. 2013, 19, 1365–1368. [Google Scholar] [CrossRef]
- Kikuchi, K.; Takakusa, H.; Nagano, T. Recent advances in the design of small molecule-based FRET sensors for cell biology. TrAC Trends Anal. Chem. 2004, 23, 407–415. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, H.J.; Yoon, S.; Park, N.; Kim, J.S. Metal Ion Induced FRET OFF-ON in Tren/Dansyl-Appended Rhodamine. Org. Lett. 2008, 10, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Haustein, E.; Jahnz, M.; Schwille, P. Triple FRET: A tool for studying long-range molecular interactions. Chem. Phys. Chem. 2003, 4, 745–748. [Google Scholar] [CrossRef]
- Efa, M.T.; Imae, T. Hybridization of carbon-dots with ZnO nanoparticles of different sizes. J. Taiwan Inst. Chem. Eng. 2018, 92, 112–117. [Google Scholar] [CrossRef]
- Efa, M.T.; Imae, T. Effects of carbon dots on ZnO nanoparticle-based dye-sensitized solar cells. Electrochim. Acta 2019, 303, 204–210. [Google Scholar] [CrossRef]
- Geleta, T.A.; Imae, T. Influence of additives on zinc oxide-based dye sensitized solar cells. Bull. Chem. Soc. Jpn. 2020, 93, 611–620. [Google Scholar] [CrossRef]
- Etefa, H.F.; Imae, T. Masatoshi Yanagida, Enhanced Photosensitization by Carbon Dots Co-adsorbing with Dye on p-Type Semiconductor (Nickel Oxide) Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 18596–18608. [Google Scholar] [CrossRef]
- Geleta, T.A.; Imae, T. Nanocomposite Photoanodes Consisting of p-NiO/n-ZnO Heterojunction and Carbon Quantum Dot Additive for Dye-Sensitized Solar Cells. ACS Appl. Nano Mater. 2021, 4, 236–249. [Google Scholar] [CrossRef]
- Imae, T.; Ikeda, S. Induced Optical Activity of Acridine Orange Bound to Poly-S-carboxymethyl-L-cysteine. Biopolymers 1971, 10, 1743–1757. [Google Scholar]
- Lohse, M.J.; Bunemann, M.; Hoffmann, C.; Vilardaga, J.P.; Nikolaev, V.O. Monitoring receptor signaling by intramolecular FRET. Curr. Opin. Pharmacol. 2007, 7, 547–553. [Google Scholar] [CrossRef]
- Sahare, P.D.; Sharma, V.K.; Mohan, D.; Rupasov, A.A. Energy transfer studies in binary dye solution mixtures: Acriflavine + Rhodamine 6G and Acriflavine + Rhodamine, B. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2008, 69, 1257–1264. [Google Scholar] [CrossRef]
- Yamanaka, T.; Takahashi, Y.; Kitamura, T.; Uchida, K. Time-resolved fluorescence spectra of pyrene doped in amorphous silica glasses. Chem. Phys. Lett. 1990, 172, 29–32. [Google Scholar] [CrossRef]
- Yang, J.-S.; Lin, C.-S.; Hwang, C.-Y. Cu2+-Induced Blue Shift of the Pyrene Excimer Emission: A New Signal Transduction Mode of Pyrene Probes. Org. Lett. 2001, 3, 889–892. [Google Scholar] [CrossRef]
- Jones, G.; Vullev, V.I. Contribution of a Pyrene Fluorescence Probe to the Aggregation Propensity of Polypeptides. Org. Lett. 2001, 3, 2457–2460. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, T.; Zhao, Y.; Fan, D.; Chen, L.; Qiu, Y.; Qian, L.; Zhang, K.; Yang, C. Crystal structure and photoluminescence of 7-(N, N′-diethylamino)-coumarin-3-carboxylic acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2008, 69, 1136–1139. [Google Scholar] [CrossRef]
- Hossain, M.A.; Mihara, H.; Ueno, A. Novel Peptides Bearing Pyrene and Coumarin Units with or without β-Cyclodextrin in Their Side Chains Exhibit Intramolecular Fluorescence Resonance Energy Transfer. J. Am. Chem. Soc. 2003, 125, 11178–11179. [Google Scholar] [CrossRef]
- Franzl, T.; Koktysh, D.S.; Klar, T.A.; Rogach, A.L.; Feldmann, J.; Gaponik, N. Fast energy transfer in layer-by-layer assembled CdTe nanocrystal bilayers. Appl. Phys. Lett. 2004, 84, 2904–2906. [Google Scholar] [CrossRef]
- Valanne, A.; Malmi, P.; Appelblom, H.; Niemela, P.; Soukka, T. A dual-step fluorescence resonance energy transfer-based quenching assay for screening of caspase-3 inhibitors. Anal. Biochem. 2008, 375, 71–81. [Google Scholar] [CrossRef]
- Lin, W.; Yuan, L.; Cao, Z.; Feng, Y.; Song, J. Through-bond energy transfer cassettes with minimal spectral overlap between the donor emission and acceptor absorption: Coumarin-rhodamine dyads with large pseudo-Stokes shifts and emission shifts. Angew. Chem. Int. Ed. Engl. 2010, 49, 375–379. [Google Scholar] [CrossRef]
- Valeur, B. Resonance Energy Transfer and Its Applications. In Molecular Fluorescence; Wiley: Weinheim, Germany, 2001; pp. 247–272. [Google Scholar]
- Valeur, B. Characteristics of Fluorescence Emission. In Molecular Fluorescence; Wiley: Weinheim, Germany, 2001; pp. 34–71. [Google Scholar]
- Takadate, A.; Masuda, T.; Murata, C.; Isobe, A.; Shinohara, T.; Irikura, M.; Goya, S. A Derivatizing Reagent-Kit Using a Single Coumarin Fluorophore. Anal. Sci. 1997, 13, 753–756. [Google Scholar] [CrossRef] [Green Version]
- Donovalova, J.; Cigan, M.; Stankovicova, H.; Gaspar, J.; Danko, M.; Gaplovsky, A.; Hrdlovic, P. Spectral properties of substituted coumarins in solution and polymer matrices. Molecules 2012, 17, 3259–3276. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Mechanisms and Dynamics of Fluorescence Quenching. In Principles of Fluorescence Spectroscopy, 3rd ed.; Lakowicz, J., Ed.; Springer: Now York, NY, USA, 2006; pp. 331–351. [Google Scholar]
- Kriegsmann, J.; Brehs, M.; Klare, J.P.; Engelhard, M.; Fitter, J. Sensory rhodopsin II/transducer complex formation in detergent and in lipid bilayers studied with FRET. Biochim. Biophys. Acta. 2009, 1788, 522–531. [Google Scholar] [CrossRef] [Green Version]
- Harriman, A.; Mallon, L.; Ziessel, R. Energy Flow in a Purpose-Built Cascade Molecule Bearing Three Distinct Chromophores Attached to the Terminal Acceptor. Chem.-Eur. J. 2008, 14, 11461–11473. [Google Scholar] [CrossRef]
- Berlman, I. Energy Transfer Parameters of Aromatic Compounds; Academic Press: New York, NY, USA, 1973. [Google Scholar]
- Lakowicz, J.R. Energy Transfer. In Principles of Fluorescence Spectroscopy, 3rd ed.; Lakowicz, J., Ed.; Springer: Now York, NY, USA, 2006; pp. 443–472. [Google Scholar]
- Michaelis, J. Quantitative Distance and Position Measurement Using Single-Molecule FRET. In Single Particle Tracking and Single Molecule Energy Transfer; Wiley: Weinheim, Germany, 2009; pp. 191–214. [Google Scholar]
- Lakowicz, J.R. Fluorescence Sensing. In Principles of Fluorescence Spectroscopy; Lakowicz, J., Ed.; Springer: Now York, NY, USA, 2006; pp. 623–673. [Google Scholar]
- Fraiji, L.K.; Hayes, D.M.; Werner, T.C. Static and dynamic fluorescence quenching experiments for the physical chemistry laboratory. J. Chem. Educ. 1992, 69, 424–428. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Quenching of Fluorescence. In Principles of Fluorescence Spectroscopy; Lakowicz, J., Ed.; Springer: Now York, NY, USA, 2006; pp. 277–330. [Google Scholar]
- Bairi, P.; Chakraborty, P.; Roy, B.; Nandi, A.K. Sensing of Hg+2 and Ag+ through a pH dependent FRET system: Fabrication of molecular logic gates. Sens. Actuators B Chem. 2014, 193, 349–355. [Google Scholar] [CrossRef]
- Stryer, L.; Haugland, R.P. Energy transfer: A spectroscopic ruler. Proc. Natl. Acad. Sci. USA 1967, 58, 719–726. [Google Scholar] [CrossRef] [Green Version]
- Ogunsolu, O.O.; Murphy, A.; Wang, J.C.; Das, A.; Hanson, K. Energy and Electron Transfer Cascade in Self-Assembled Bilayer Dye Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 28633–28640. [Google Scholar] [CrossRef]
- Lin, Y.J.; Chen, J.W.; Hsiao, P.T.; Tung, Y.L.; Chang, C.C.; Chen, C.M. Efficiency improvement of dye-sensitized solar cells by in situ fluorescence resonance energy transfer. J. Mater. Chem. A 2017, 5, 9081–9089. [Google Scholar] [CrossRef]
- Gibson, E.A.; Smeigh, A.L.; Pleux, L.L.; Fortage, J.; Boschloo, G.; Blart, E.; Pellegrin, Y.; Odobel, F.; Hagfeldt, A.; Hammarstrm, L. A p-Type NiO-Based Dye-Sensitized Solar Cell with an Open-Circuit Voltage of 0.35 V. Angew. Chem. Int. Ed. 2009, 48, 4402–4405. [Google Scholar] [CrossRef]
- Freys, J.C.; Gardner, J.M.; Amario, L.D.; Brown, A.M.; Hammarström, L. Ru-based donor–acceptor photosensitizer that retards charge recombination in a p-type dye-sensitized solar cell. Dalton Trans. 2012, 41, 13105–13111. [Google Scholar] [CrossRef]
- Mor, G.K.; Basham, J.; Paulose, M.; Kim, S.; Varghese, O.K.; Vaish, A.; Yoriya, S.; Grimes, C.A. High-Efficiency Förster Resonance Energy Transfer in Solid-State Dye Sensitized Solar Cells. Nano Lett. 2010, 10, 2387–2394. [Google Scholar] [CrossRef]
- Kim, D.W.; Jo, H.-J.; Thogiti, S.; Yang, W.K.; Cheruku, R.; Kim, J.H. Fluorescent material concentration dependency: Förster resonance energy transfer in quasi-solid state DSSCs. Elect. Materi. Lett. 2017, 13, 249–254. [Google Scholar] [CrossRef]
- Nakhaei, R.; Razeghizadeh, A.; Shabani, P.; Ganji, J.; Tabatabaee, S.S. Combination of co-sensitization and Förster resonance energy transfer in natural-synthetic dye sensitized solar cells. Opt. Mater. 2022, 131, 112690. [Google Scholar] [CrossRef]
- Seok, S.; Goud, B.S.; Gwak, S.J.; Chitumalla, R.K.; Lim, J.; Lee, W.; Thuy, C.T.T.; Vuppala, S.; Jang, J.; Koyyada, G.; et al. Unveiling the effect of TADF as an energy relay dye in fluorescence resonance energy transfer based solid-state dye-sensitized solar cells. J. Mol. Struc. 2022, 1249, 131576. [Google Scholar]
- Ramanarayanan, R.; Swaminathan, S. Improving efficiency of natural dye sensitized solar cell using FRET mechanism. Mater. Today Proc. 2022, 60, 1125–1129. [Google Scholar] [CrossRef]





 : Py(3.5 μM)-AC(4.9 mM)-AO(4.9 mM),
: Py(3.5 μM)-AC(4.9 mM)-AO(4.9 mM),  : Py(5 μM)-AC(7 mM)-AO(7 mM), and
: Py(5 μM)-AC(7 mM)-AO(7 mM), and  : Py(6.5 μM)-AC(9.1 mM)-AO (9.1 mM).
: Py(6.5 μM)-AC(9.1 mM)-AO (9.1 mM).
 : Py(3.5 μM)-AC(4.9 mM)-AO(4.9 mM),
: Py(3.5 μM)-AC(4.9 mM)-AO(4.9 mM),  : Py(5 μM)-AC(7 mM)-AO(7 mM), and
: Py(5 μM)-AC(7 mM)-AO(7 mM), and  : Py(6.5 μM)-AC(9.1 mM)-AO (9.1 mM).
: Py(6.5 μM)-AC(9.1 mM)-AO (9.1 mM).


| Electrode Material | JSC (mA/cm2) | Voc (V) | FF | PCE (%) |
|---|---|---|---|---|
| ZnO@Cdot | 0.006 ± 0.001 | 0.17 ± 0.10 | 0.20 ± 0.01 | 0.001 ± 0.000 |
| ZnO@Cdot+(Py-AC-AO) | 1.30 ± 0.01 | 0.15 ± 0.03 | 0.17 ± 0.02 | 0.21 ± 0.01 |
| ZnO@N719 | 0.61 ± 0.10 | 0.32 ± 0.02 | 0.65 ± 0.01 | 0.80 ± 0.01 |
| ZnO@N719+Py | 1.73 ± 0.07 | 0.83 ± 0.06 | 0.31 ± 0.01 | 2.80 ± 0.18 |
| ZnO@N719+(Py-AC) | 2.61 ± 0.01 | 0.95 ± 0.05 | 0.28 ± 0.02 | 4.45 ± 0.15 |
| ZnO@N719+(Py-AC-AO) | 2.85 ± 0.02 | 0.95 ± 0.11 | 0.29 ± 0.02 | 4.61 ± 0.20 |
| ZnO@Cdot@N719 | 2.34 ± 0.03 | 0.67 ± 0.10 | 0.59 ± 0.05 | 5.92 + 0.01 |
| ZnO@Cdot@N719+Py | 3.25 ± 0.09 | 0.81+0.14 | 0.37 ± 0.11 | 6.24 + 0.13 |
| ZnO@Cdot@N719+(Py-AC) | 3.82 ± 0.03 | 0.79 ± 0.12 | 0.38 ± 0.02 | 7.31 ± 0.10 |
| ZnO@Cdot@N719+(Py-AC-AO(14.3wt%)) | 6.10 ± 0.03 | 0.77 ± 0.30 | 0.30 ± 0.05 | 9.03 ± 0.14 |
| ZnO@Cdot@N719+(Py-AC-AO(16.7wt%)) | 6.44 ± 0.01 | 0.79 ± 0.20 | 0.32 ± 0.03 | 10.37 ± 0.12 |
| ZnO@Cdot@N719+(Py-AC-AO(20.0wt%)) | 5.40 ± 0.04 | 0.77 + 0.11 | 0.30 ± 0.11 | 7.99 ± 0.13 |
| ZnO@N719+Py+C6 | 2.00 ± 0.01 | 0.78 ± 0.07 | 0.31 ± 0.09 | 3.10 ± 0.33 |
| ZnO@N719+Py+(C6-AO) | 2.50 ± 0.03 | 0.79 ± 0.21 | 0.30 ± 0.02 | 3.79 ± 0.23 |
| ZnO@Cdot@N719+Py | 3.25 ± 0.09 | 0.81 ± 0.14 | 0.37 ± 0.11 | 6.24 ± 0.13 |
| ZnO@Cdot@N719+(Py-C6) | 3.50 ± 0.11 | 0.83 ± 0.22 | 0.38 ± 0.02 | 7.01 ± 0.21 |
| ZnO@Cdot@N719+(Py-C6-AO(14.3wt%)) | 3.81 ± 0.02 | 0.92 ± 0.12 | 0.37+0.02 | 8.35 ± 0.14 |
| ZnO@Cdot@N719+(Py-C6-AO(16.7wt%)) | 3.94 ± 0.11 | 0.93 ± 0.20 | 0.41 ± 0.01 | 9.63 ± 0.11 |
| ZnO@Cdot@N719+(Py-C6-AO(20.0wt%)) | 3.61 ± 0.11 | 0.81 ± 0.01 | 0.36 ± 0.02 | 6.74 ± 0.22 |
| NiO@N719+Py | 2.85 ± 0.02 | 0.45 ± 0.10 | 0.22 ± 0.03 | 1.84 ± 0.10 |
| NiO@N719+(Py-C6) | 3.42 ± 0.01 | 0.45 ± 0.15 | 0.23 ± 0.06 | 2.25 ± 0.22 |
| NiO@N719+(Py-C6-AO) | 4.10 ± 0.05 | 0.38 ± 0.32 | 0.26 ± 0.02 | 2.59 ± 0.24 |
| NiO@Cdot@N719+Py | 8.13 ± 0.03 | 0.65 ± 0.13 | 0.20 ± 0.01 | 6.75 ± 0.15 |
| NiO@Cdot@N719+(Py-C6) | 10.31 ± 0.01 | 0.62 ± 0.20 | 0.21 ± 0.04 | 8.34 ± 0.10 |
| NiO@Cdot@N719+(Py-C6-AO) | 13.95 ± 0.05 | 0.62 ± 0.16 | 0.22 ± 0.02 | 11.36 ± 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efa, M.T.; Huang, J.-C.; Imae, T. Cascade Förster Resonance Energy Transfer Studies for Enhancement of Light Harvesting on Dye-Sensitized Solar Cells. Nanomaterials 2022, 12, 4085. https://doi.org/10.3390/nano12224085
Efa MT, Huang J-C, Imae T. Cascade Förster Resonance Energy Transfer Studies for Enhancement of Light Harvesting on Dye-Sensitized Solar Cells. Nanomaterials. 2022; 12(22):4085. https://doi.org/10.3390/nano12224085
Chicago/Turabian StyleEfa, Mulugeta Tesema, Jheng-Chang Huang, and Toyoko Imae. 2022. "Cascade Förster Resonance Energy Transfer Studies for Enhancement of Light Harvesting on Dye-Sensitized Solar Cells" Nanomaterials 12, no. 22: 4085. https://doi.org/10.3390/nano12224085