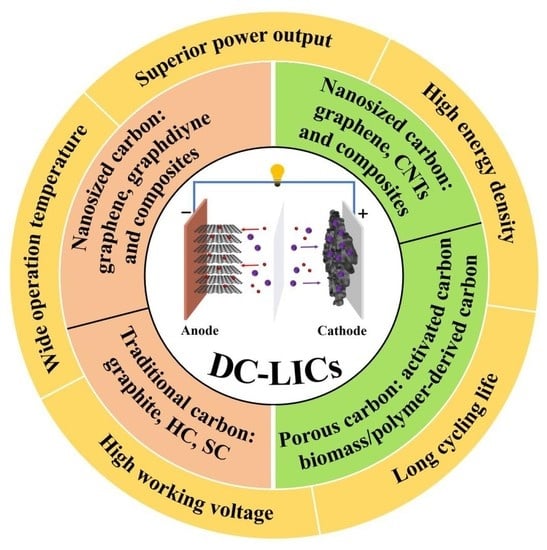
Advances of Carbon Materials for Dual-Carbon Lithium-Ion Capacitors: A Review
Abstract
:1. Introduction
2. Progresses of Carbon Materials as Cathode for DC-LICs
2.1. Traditional Porous Carbon Cathode
2.1.1. Activated Carbon
2.1.2. Biomass-Derived Porous Carbon
2.1.3. Polymer-Derived Porous Carbon
2.2. Nanosized Carbon-Based Cathode
2.2.1. Graphene-Based Cathode
2.2.2. Carbon Nanotube-Based Cathode
3. Progresses of Carbon Anode Materials for DC-LICs
3.1. Traditional Carbon Anode
3.1.1. Graphite
3.1.2. Hard/Soft Carbon
3.2. Nanosized Carbon-Based Anode
3.2.1. Graphene-Based Anode
3.2.2. Graphdiyne-Based Anode
4. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [Green Version]
- Yadlapalli, R.T.; Alla, R.R.; Kandipati, R.; Kotapati, A. Super capacitors for energy storage: Progress, applications and challenges. J. Energy Storage 2022, 49, 104194. [Google Scholar] [CrossRef]
- Liu, G.; Li, M.; Wu, N.; Cui, L.; Huang, X.; Liu, X.; Zhao, Y.; Chen, H.; Yuan, W.; Bai, Y. Single-Crystalline Particles: An Effective Way to Ameliorate the Intragranular Cracking, Thermal Stability, and Capacity Fading of the LiNi0.6Co0.2Mn0.2O2 Electrodes. J. Electrochem. Soc. 2018, 165, A3040–A3047. [Google Scholar] [CrossRef]
- Li, H.; Guo, C.; Zhang, T.; Xue, P.; Zhao, R.; Zhou, W.; Li, W.; Elzatahry, A.; Zhao, D.; Chao, D. Hierarchical Confinement Effect with Zincophilic and Spatial Traps Stabilized Zn-Based Aqueous Battery. Nano Lett. 2022, 22, 4223–4231. [Google Scholar] [CrossRef]
- Guo, D.; Wang, F.; Yang, M.; Hu, G.; Liu, G.; Wu, N.; Qin, A.; Liu, X. Constructing abundant oxygen vacancies in NaVPO4F@C for boosting sodium storage kinetics. Electrochim. Acta 2022, 424, 140695. [Google Scholar] [CrossRef]
- Sui, D.; Chang, M.; Wang, H.; Qian, H.; Yang, Y.; Li, S.; Zhang, Y.; Song, Y. A Brief Review of Catalytic Cathode Materials for Na-CO2 Batteries. Catalysts 2021, 11, 603. [Google Scholar] [CrossRef]
- Gopalakrishnan, R.; Goutam, S.; Miguel Oliveira, L.; Timmermans, J.-M.; Omar, N.; Messagie, M.; Van den Bossche, P.; van Mierlo, J. A Comprehensive Study on Rechargeable Energy Storage Technologies. J. Electrochem. Energy Convers. Storage 2017, 13, 040801. [Google Scholar] [CrossRef]
- Poizot, P.; Dolhem, F. Clean energy new deal for a sustainable world: From non-CO2 generating energy sources to greener electrochemical storage devices. Energy Environ. Sci. 2011, 4, 2003–2019. [Google Scholar] [CrossRef]
- Yan, W.B.; Sui, D.; Yang, Y.L.; Chang, M.J. Bi- or multi-functional charge transporting materials for perovskite solar cells. Dye. Pigment. 2022, 200, 110102. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Liu, G.; Xiao, F.; Zhang, T.; Gu, Y.; Li, J.; Guo, D.; Xu, M.; Wu, N.; Cao, A.; Liu, X. In-situ growth of MoO2@N doped carbon on Mo2C-MXene for superior lithium storage. Appl. Surf. Sci. 2022, 597, 153688. [Google Scholar] [CrossRef]
- Wu, N.; Wu, H.; Kim, J.-K.; Liu, X.; Zhang, Y. Restoration of Degraded Nickel-Rich Cathode Materials for Long-Life Lithium-Ion Batteries. ChemElectroChem 2018, 5, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, F.; Yang, X.; Long, G.; Wu, Y.; Zhang, T.; Leng, K.; Huang, Y.; Ma, Y.; Yu, A.; et al. Porous 3D graphene-based bulk materials with exceptional high surface area and excellent conductivity for supercapacitors. Sci. Rep. 2013, 3, 1408. [Google Scholar] [CrossRef] [Green Version]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, X.; Sarkar, S.; Zhao, Y. Challenges and opportunities for supercapacitors. APL Mater. 2019, 7, 100901. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Wang, D.-W. Design Rationale and Device Configuration of Lithium-Ion Capacitors. Adv. Energy Mater. 2022, 12, 2200920. [Google Scholar] [CrossRef]
- Li, B.; Zheng, J.; Zhang, H.; Jin, L.; Yang, D.; Lv, H.; Shen, C.; Shellikeri, A.; Zheng, Y.; Gong, R.; et al. Electrode Materials, Electrolytes, and Challenges in Nonaqueous Lithium-Ion Capacitors. Adv. Mater. 2018, 30, 1705670. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, F.; Zhang, L.; Lu, Y.; Zhang, Y.; Yang, X.; Ma, Y.; Huang, Y. High energy density Li-ion capacitor assembled with all graphene-based electrodes. Carbon 2015, 92, 106–118. [Google Scholar] [CrossRef]
- Lang, J.; Zhang, X.; Liu, B.; Wang, R.; Chen, J.; Yan, X. The roles of graphene in advanced Li-ion hybrid supercapacitors. J. Energy Chem. 2018, 27, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Aravindan, V.; Gnanaraj, J.; Lee, Y.-S.; Madhavi, S. Insertion-Type Electrodes for Nonaqueous Li-Ion Capacitors. Chem. Rev. 2014, 114, 11619–11635. [Google Scholar] [CrossRef]
- Jagadale, A.; Zhou, X.; Xiong, R.; Dubal, D.P.; Xu, J.; Yang, S. Lithium ion capacitors (LICs): Development of the materials. Energy Storage Mater. 2019, 19, 314–329. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Niu, Z. Carbon-based materials for lithium-ion capacitors. Mater. Chem. Front. 2019, 3, 1265–1279. [Google Scholar] [CrossRef]
- Sui, D.; Chang, M.; Peng, Z.; Li, C.; He, X.; Yang, Y.; Liu, Y.; Lu, Y. Graphene-Based Cathode Materials for Lithium-Ion Capacitors: A Review. Nanomaterials 2021, 11, 2771. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chang, H.; Zhang, M.; Chen, Y. Graphene-Based Materials for Lithium-Ion Hybrid Supercapacitors. Adv. Mater. 2015, 27, 5296–5308. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Sun, C.; Wang, K.; Sun, X.; Ma, Y. Recent progress of graphene-based materials in lithium-ion capacitors. J. Phys. D Appl. Phys. 2019, 52, 143001. [Google Scholar] [CrossRef]
- Si, L.; Yan, K.; Li, C.; Huang, Y.; Pang, X.; Yang, X.; Sui, D.; Zhang, Y.; Wang, J.; Charles Xu, C. Binder-free SiO2 nanotubes/carbon nanofibers mat as superior anode for lithium-ion batteries. Electrochim. Acta 2022, 404, 139747. [Google Scholar] [CrossRef]
- Wu, N.; Qiao, X.; Shen, J.; Liu, G.; Sun, T.; Wu, H.; Hou, H.; Liu, X.; Zhang, Y.; Ji, X. Anatase inverse opal TiO2-x@N-doped C induced the dominant pseudocapacitive effect for durable and fast lithium/sodium storage. Electrochim. Acta 2019, 299, 540–548. [Google Scholar] [CrossRef]
- Guo, D.; Yang, M.; Zhang, L.; Li, Y.; Wang, J.; Liu, G.; Wu, N.; Kim, J.-K.; Liu, X. Cr2O3 nanosheet/carbon cloth anode with strong interaction and fast charge transfer for pseudocapacitive energy storage in lithium-ion batteries. RSC Adv. 2019, 9, 33446–33453. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Geng, L.; Yi, S.; Li, C.; An, Y.; Zhang, X.; Zhang, X.; Wang, K.; Ma, Y. Effects of carbon black on the electrochemical performances of SiOx anode for lithium-ion capacitors. J. Power Sources 2021, 499, 229936. [Google Scholar] [CrossRef]
- Leng, K.; Zhang, F.; Zhang, L.; Zhang, T.; Wu, Y.; Lu, Y.; Huang, Y.; Chen, Y. Graphene-based Li-ion hybrid supercapacitors with ultrahigh performance. Nano Res. 2013, 6, 581–592. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Zheng, W.; Zhao, Q.; Sun, S.; Ji, G.; Li, S.; Fan, X.; Xu, C. Design of Nb2O5/graphene hybrid aerogel as polymer binder-free electrodes for lithium-ion capacitors. Mater. Technol. 2020, 35, 625–634. [Google Scholar] [CrossRef]
- Yang, C.; Lan, J.-L.; Ding, C.; Wang, F.; Siyal, S.H.; Yu, Y.; Yang, X. Three-dimensional hierarchical ternary aerogels of ultrafine TiO2 nanoparticles@porous carbon nanofibers-reduced graphene oxide for high-performance lithium-ion capacitors. Electrochim. Acta 2019, 296, 790–798. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, T.; Yang, X.; Zhang, L.; Leng, K.; Huang, Y.; Chen, Y. A high-performance supercapacitor-battery hybrid energy storage device based on graphene-enhanced electrode materials with ultrahigh energy density. Energy Environ. Sci. 2013, 6, 1623–1632. [Google Scholar] [CrossRef]
- Wang, R.; Wang, S.; Jin, D.; Zhang, Y.; Cai, Y.; Ma, J.; Zhang, L. Engineering layer structure of MoS2-graphene composites with robust and fast lithium storage for high-performance Li-ion capacitors. Energy Storage Mater. 2017, 9, 195–205. [Google Scholar] [CrossRef]
- Shao, R.; Niu, J.; Zhu, F.; Dou, M.; Zhang, Z.; Wang, F. A facile and versatile strategy towards high-performance Si anodes for Li-ion capacitors: Concomitant conductive network construction and dual-interfacial engineering. Nano Energy 2019, 63, 103824. [Google Scholar] [CrossRef]
- Liu, C.; Ren, Q.; Zhang, S.; Yin, B.-S.; Que, L.; Zhao, L.; Sui, X.; Yu, F.; Li, X.; Gu, D.; et al. High energy and power lithium-ion capacitors based on Mn3O4/3D-graphene as anode and activated polyaniline-derived carbon nanorods as cathode. Chem. Eng. J. 2019, 370, 1485–1492. [Google Scholar] [CrossRef]
- Sui, D.; Si, L.; Li, C.; Yang, Y.; Zhang, Y.; Yan, W. A Comprehensive Review of Graphene-Based Anode Materials for Lithium-ion Capacitors. Chemistry 2021, 3, 1215–1246. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Xu, Y.; Wang, L.; Li, Z.; Li, C.; Wang, K.; Sun, X.; An, Y.; Wu, Z.-S.; et al. 2D Graphene/MnO Heterostructure with Strongly Stable Interface Enabling High-Performance Flexible Solid-State Lithium-Ion Capacitors. Adv. Funct. Mater. 2022, 32, 2202342. [Google Scholar] [CrossRef]
- Guo, D.; Yang, M.; Wang, F.; Cheng, Y.; Zhang, A.; Liu, G.; Wu, N.; Cao, A.; Mi, H.; Liu, X. Regulating the electronic structure of MoO2/Mo2C/C by heterostructure and oxygen vacancies for boosting lithium storage kinetics. Dalton Trans. 2022, 51, 12620–12629. [Google Scholar] [CrossRef]
- Cao, W.J.; Zheng, J.P. Li-ion capacitors with carbon cathode and hard carbon/stabilized lithium metal powder anode electrodes. J. Power Sources 2012, 213, 180–185. [Google Scholar] [CrossRef]
- Wang, H.; Shao, Y.; Mei, S.; Lu, Y.; Zhang, M.; Sun, J.-K.; Matyjaszewski, K.; Antonietti, M.; Yuan, J. Polymer-Derived Heteroatom-Doped Porous Carbon Materials. Chem. Rev. 2020, 120, 9363–9419. [Google Scholar] [CrossRef] [PubMed]
- Sennu, P.; Aravindan, V.; Ganesan, M.; Lee, Y.-G.; Lee, Y.-S. Biomass-Derived Electrode for Next Generation Lithium-Ion Capacitors. ChemSusChem 2016, 9, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Li, H.; Shi, R.; Xu, L.; Li, J.; Kang, F.; Li, B. Nanostructured Anode Materials for Non-aqueous Lithium Ion Hybrid Capacitors. Energy Environ. Mater. 2018, 1, 75–87. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Xu, Y.; Li, C.; Wang, K.; Sun, X.; Su, F.; Chen, C.-M.; Liu, F.; Wu, Z.-S.; et al. Recent Advances on Carbon-Based Materials for High Performance Lithium-Ion Capacitors. Batter. Supercaps 2021, 4, 407–428. [Google Scholar] [CrossRef]
- Su, F.; Hou, X.; Qin, J.; Wu, Z.-S. Recent Advances and Challenges of Two-Dimensional Materials for High-Energy and High-Power Lithium-Ion Capacitors. Batter. Supercaps 2020, 3, 10–29. [Google Scholar] [CrossRef] [Green Version]
- Hou, R.; Liu, B.; Sun, Y.; Liu, L.; Meng, J.; Levi, M.D.; Ji, H.; Yan, X. Recent advances in dual-carbon based electrochemical energy storage devices. Nano Energy 2020, 72, 104728. [Google Scholar] [CrossRef]
- Li, F.; Cao, Y.; Wu, W.; Wang, G.; Qu, D. Prelithiation Bridges the Gap for Developing Next-Generation Lithium-Ion Batteries/Capacitors. Small Methods 2022, 6, 2200411. [Google Scholar] [CrossRef]
- Zhang, S.S. Dual-Carbon Lithium-Ion Capacitors: Principle, Materials, and Technologies. Batter. Supercaps 2020, 3, 1137–1146. [Google Scholar] [CrossRef]
- Li, G.; Yang, Z.; Yin, Z.; Guo, H.; Wang, Z.; Yan, G.; Liu, Y.; Li, L.; Wang, J. Non-aqueous dual-carbon lithium-ion capacitors: A review. J. Mater. Chem. A 2019, 7, 15541–15563. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, H.; Li, X.; Wang, Z.; Yan, Z.; Wang, Y. Natural sisal fibers derived hierarchical porous activated carbon as capacitive material in lithium ion capacitor. J. Power Sources 2016, 329, 339–346. [Google Scholar] [CrossRef]
- Qiu, D.; Kang, C.; Li, M.; Wei, J.; Hou, Z.; Wang, F.; Yang, R. Biomass-derived mesopore-dominant hierarchical porous carbon enabling ultra-efficient lithium ion storage. Carbon 2020, 162, 595–603. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Wang, K.; Sun, X.; Ma, Y. High-power and long-life lithium-ion capacitors constructed from N-doped hierarchical carbon nanolayer cathode and mesoporous graphene anode. Carbon 2018, 140, 237–248. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, H.; Yan, G.; Li, X.; Wang, Z.; Guo, Y.; Wang, X.; Wu, Y.; Wang, J. High-Value Utilization of Lignin To Prepare Functional Carbons toward Advanced Lithium-Ion Capacitors. ACS Sustain. Chem. Eng. 2020, 8, 11522–11531. [Google Scholar] [CrossRef]
- Huang, X.; Le, T.; Chen, M.; Yang, C.; Yang, Y. Regulating the Pore Structure and Heteroatom Doping of Hollow Carbon Fiber Based on a Trifunctional Template Method for High-Performance Lithium-Ion Capacitors. ACS Appl. Energy Mater. 2022, 5, 3034–3042. [Google Scholar] [CrossRef]
- Xia, Q.; Yang, H.; Wang, M.; Yang, M.; Guo, Q.; Wan, L.; Xia, H.; Yu, Y. High Energy and High Power Lithium-Ion Capacitors Based on Boron and Nitrogen Dual-Doped 3D Carbon Nanofibers as Both Cathode and Anode. Adv. Energy Mater. 2017, 7, 1701336. [Google Scholar] [CrossRef]
- Hao, J.; Bai, J.; Wang, X.; Wang, Y.; Guo, Q.; Yang, Y.; Zhao, J.; Chi, C.; Li, Y. S, O dual-doped porous carbon derived from activation of waste papers as electrodes for high performance lithium ion capacitors. Nanoscale Adv. 2021, 3, 738–746. [Google Scholar] [CrossRef]
- Han, L.; Kang, S.; Zhu, X.; Li, J.; Wang, Q.; Jia, X. High-Performance Lithium-Ion Capacitors Produced by Atom-Thick Carbon Cathode and Nitrogen-Doped Porous Carbon Anode. Energy Fuels 2021, 35, 16894–16902. [Google Scholar] [CrossRef]
- Chen, M.; Le, T.; Zhou, Y.; Kang, F.; Yang, Y. Thiourea-Induced N/S Dual-Doped Hierarchical Porous Carbon Nanofibers for High-Performance Lithium-Ion Capacitors. ACS Appl. Energy Mater. 2020, 3, 1653–1664. [Google Scholar] [CrossRef]
- Liang, T.; Wang, H.; Fei, R.; Wang, R.; He, B.; Gong, Y.; Yan, C. A high-power lithium-ion hybrid capacitor based on a hollow N-doped carbon nanobox anode and its porous analogue cathode. Nanoscale 2019, 11, 20715–20724. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, J.; Li, Z.; Yang, Z.; Zhang, Y.; An, Y.; Zhu, Q.; Dou, H.; Zhang, X. Aerosol-assisted preparation of N-doped hierarchical porous carbon spheres cathodes toward high-stable lithium-ion capacitors. J. Mater. Sci. 2020, 55, 13127–13140. [Google Scholar] [CrossRef]
- Li, Z.; Cao, L.; Chen, W.; Huang, Z.; Liu, H. Mesh-Like Carbon Nanosheets with High-Level Nitrogen Doping for High-Energy Dual-Carbon Lithium-Ion Capacitors. Small 2019, 15, 1805173. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Nie, P.; Fang, S.; Zhang, Y.; An, Y.; Fu, R.; Dou, H.; Zhang, X. Boron and nitrogen dual-doped carbon as a novel cathode for high performance hybrid ion capacitors. Chin. Chem. Lett. 2018, 29, 624–628. [Google Scholar] [CrossRef]
- Sun, F.; Liu, X.; Wu, H.B.; Wang, L.; Gao, J.; Li, H.; Lu, Y. In Situ High-Level Nitrogen Doping into Carbon Nanospheres and Boosting of Capacitive Charge Storage in Both Anode and Cathode for a High-Energy 4.5 V Full-Carbon Lithium-Ion Capacitor. Nano Lett. 2018, 18, 3368–3376. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Deng, Y.; Wu, W.; Zhang, S.; Chen, G. A novel eutectic solvent precursor for efficiently preparing N-doped hierarchically porous carbon nanosheets with unique surface functional groups and micropores towards dual-carbon lithium-ion capacitors. J. Mater. Chem. A 2021, 9, 13631–13641. [Google Scholar] [CrossRef]
- Chen, M.; Le, T.; Zhou, Y.; Kang, F.; Yang, Y. Enhanced Electrode Matching Assisted by In Situ Etching and Co-Doping toward High-Rate Dual-Carbon Lithium-Ion Capacitors. ACS Sustain. Chem. Eng. 2021, 9, 10054–10061. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, W.H.; Ryou, M.-H.; Jin, J.K.; Kim, J.; Choi, J.W. Functionalized Graphene for High Performance Lithium Ion Capacitors. ChemSusChem 2012, 5, 2328–2333. [Google Scholar] [CrossRef]
- Dubal, D.P.; Gomez-Romero, P. All nanocarbon Li-Ion capacitor with high energy and high power density. Mater. Today Energy 2018, 8, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhang, X.; Wang, K.; Sun, X.; Xu, Y.; Su, F.; Chen, C.-M.; Liu, F.; Wu, Z.-S.; Ma, Y. Nitrogen-enriched graphene framework from a large-scale magnesiothermic conversion of CO2 with synergistic kinetics for high-power lithium-ion capacitors. NPG Asia Mater. 2021, 13, 59. [Google Scholar] [CrossRef]
- Xiao, Z.; Yu, Z.; Gao, Z.; Li, B.; Zhang, M.; Xu, C. S-doped graphene nano-capsules toward excellent low-temperature performance in Li-ion capacitors. J. Power Sources 2022, 535, 231404. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, Q.; Zheng, W.; Ren, Z.; Hu, X.; Li, J.; Lu, L.; Hu, N.; Molenda, J.; Liu, X.; et al. Achieving high energy density in a 4.5 V all nitrogen-doped graphene based lithium-ion capacitor. J. Mater. Chem. A 2019, 7, 19909–19921. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, J.; He, D.; Chen, S.; Peng, W.; Hu, X.; Liu, T.; Zhu, Z.; Bai, Y. Facile Synthesis of Graphene with Fast Ion/Electron Channels for High-Performance Symmetric Lithium-Ion Capacitors. ACS Appl. Mater. Interfaces 2021, 13, 38266–38277. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Cai, P.; Wang, B.; Liu, C.; Li, J.; Qiu, T.; Zou, G.; Hou, H.; Ji, X. Insights into Enhanced Capacitive Behavior of Carbon Cathode for Lithium Ion Capacitors: The Coupling of Pore Size and Graphitization Engineering. Nano-Micro Lett. 2020, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Amatucci, G.G.; Badway, F.; Du Pasquier, A.; Zheng, T. An Asymmetric Hybrid Nonaqueous Energy Storage Cell. J. Electrochem. Soc. 2001, 148, A930. [Google Scholar] [CrossRef]
- Sivakkumar, S.R.; Pandolfo, A.G. Evaluation of lithium-ion capacitors assembled with pre-lithiated graphite anode and activated carbon cathode. Electrochim. Acta 2012, 65, 280–287. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Wang, J.; Shi, J.; Shi, Z. Different types of pre-lithiated hard carbon as negative electrode material for lithium-ion capacitors. Electrochim. Acta 2016, 187, 134–142. [Google Scholar] [CrossRef]
- Ren, J.J.; Su, L.W.; Qin, X.; Yang, M.; Wei, J.P.; Zhou, Z.; Shen, P.W. Pre-lithiated graphene nanosheets as negative electrode materials for Li-ion capacitors with high power and energy density. J. Power Sources 2014, 264, 108–113. [Google Scholar] [CrossRef]
- An, Y.; Liu, T.; Li, C.; Zhang, X.; Hu, T.; Sun, X.; Wang, K.; Wang, C.; Ma, Y. A general route for the mass production of graphene-enhanced carbon composites toward practical pouch lithium-ion capacitors. J. Mater. Chem. A 2021, 9, 15654–15664. [Google Scholar] [CrossRef]
- Li, B.; Xiao, Z.; Chen, M.; Huang, Z.; Tie, X.; Zai, J.; Qian, X. Rice husk-derived hybrid lithium-ion capacitors with ultra-high energy. J. Mater. Chem. A 2017, 5, 24502–24507. [Google Scholar] [CrossRef]
- Dubey, P.; Shrivastav, V.; Maheshwari, P.H.; Sundriyal, S. Recent advances in biomass derived activated carbon electrodes for hybrid electrochemical capacitor applications: Challenges and opportunities. Carbon 2020, 170, 1–29. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, X.; Wang, L.; Fu, H. Recent advances of biomass derived carbon-based materials for efficient electrochemical energy devices. J. Mater. Chem. A 2022, 10, 9277–9307. [Google Scholar] [CrossRef]
- Luo, X.; Chen, S.; Hu, T.; Chen, Y.; Li, F. Renewable biomass-derived carbons for electrochemical capacitor applications. SusMat 2021, 1, 211–240. [Google Scholar] [CrossRef]
- Sennu, P.; Arun, N.; Madhavi, S.; Aravindan, V.; Lee, Y.-S. All carbon based high energy lithium-ion capacitors from biomass: The role of crystallinity. J. Power Sources 2019, 414, 96–102. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Yang, X.; Leng, K.; Huang, Y.; Chen, Y. High-Performance Supercapacitor Electrode Materials Prepared from Various Pollens. Small 2013, 9, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Chang-Jian, C.-W.; Hsieh, T.-H.; Huang, J.-H.; Chu Weng, H.; Hsiao, Y.-S.; Syu, W.-L.; Chen, C.-P. High-performance Li-Ion capacitor constructed from biomass-derived porous carbon and high-rate Li4Ti5O12. Appl. Surf. Sci. 2021, 543, 148717. [Google Scholar] [CrossRef]
- Li, B.; Zhang, H.; Zhang, C. Agricultural waste-derived activated carbon/graphene composites for high performance lithium-ion capacitors. RSC Adv. 2019, 9, 29190–29194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Zhang, L.; Sun, J.; Li, K.; Zhao, S.; Zhao, D.; Wang, J.; Yang, C.; Wang, X.; Cao, B. Corncob-Derived Hierarchical Porous Activated Carbon for High-Performance Lithium-Ion Capacitors. Energy Fuels 2020, 34, 16885–16892. [Google Scholar] [CrossRef]
- Sun, J.; Yang, S.; Ai, J.; Yang, C.; Jia, Q.; Cao, B. Hierarchical Porous Activated Carbon Obtained by a Novel Heating-Rate-Induced Method for Lithium-Ion Capacitor. ChemistrySelect 2019, 4, 5300–5307. [Google Scholar] [CrossRef]
- Babu, B.; Lashmi, P.G.; Shaijumon, M.M. Li-ion capacitor based on activated rice husk derived porous carbon with improved electrochemical performance. Electrochim. Acta 2016, 211, 289–296. [Google Scholar] [CrossRef]
- Lu, Q.; Lu, B.; Chen, M.; Wang, X.; Xing, T.; Liu, M.; Wang, X. Porous activated carbon derived from Chinese-chive for high energy hybrid lithium-ion capacitor. J. Power Sources 2018, 398, 128–136. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, X.; Wang, H.; Lyu, Y.; Liu, X.; Zhao, Y.; Shi, J.; Liu, W.; Paek, E.; Mitlin, D. Lithium Ion Capacitor with Identical Carbon Electrodes Yields 6 s Charging and 100 000 Cycles Stability with 1% Capacity Fade. ACS Sustain. Chem. Eng. 2019, 7, 2867–2877. [Google Scholar] [CrossRef]
- Ajuria, J.; Zarrabeitia, M.; Arnaiz, M.; Urra, O.; Rojo, T.; Goikolea, E. Graphene as Vehicle for Ultrafast Lithium Ion Capacitor Development Based on Recycled Olive Pit Derived Carbons. J. Electrochem. Soc. 2019, 166, A2840–A2848. [Google Scholar] [CrossRef]
- Gómez-Urbano, J.L.; Moreno-Fernández, G.; Arnaiz, M.; Ajuria, J.; Rojo, T.; Carriazo, D. Graphene-coffee waste derived carbon composites as electrodes for optimized lithium ion capacitors. Carbon 2020, 162, 273–282. [Google Scholar] [CrossRef]
- Zheng, B.; Lin, X.; Zhang, X.; Wu, D.; Matyjaszewski, K. Emerging Functional Porous Polymeric and Carbonaceous Materials for Environmental Treatment and Energy Storage. Adv. Funct. Mater. 2020, 30, 1907006. [Google Scholar] [CrossRef]
- Abdelaal, M.M.; Hung, T.-C.; Mohamed, S.G.; Yang, C.-C.; Hung, T.-F. Two Birds with One Stone: Hydrogel-Derived Hierarchical Porous Activated Carbon toward the Capacitive Performance for Symmetric Supercapacitors and Lithium-Ion Capacitors. ACS Sustain. Chem. Eng. 2022, 10, 4717–4727. [Google Scholar] [CrossRef]
- Zheng, W.; Li, Z.; Han, G.; Zhao, Q.; Lu, G.; Hu, X.; Sun, J.; Wang, R.; Xu, C. Nitrogen-doped activated porous carbon for 4.5 V lithium-ion capacitor with high energy and power density. J. Energy Storage 2022, 47, 103675. [Google Scholar] [CrossRef]
- Arnaiz, M.; Nair, V.; Mitra, S.; Ajuria, J. Furfuryl alcohol derived high-end carbons for ultrafast dual carbon lithium ion capacitors. Electrochim. Acta 2019, 304, 437–446. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, S.; Xu, H.; Nagai, A.; Jiang, D. Conjugated microporous polymers: Design, synthesis and application. Chem. Soc. Rev. 2013, 42, 8012–8031. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef]
- Weng, T.-H.; Mohamed, M.G.; Sharma, S.U.; Chaganti, S.V.; Samy, M.M.; Lee, J.-T.; Kuo, S.-W. Ultrastable Three-Dimensional Triptycene- and Tetraphenylethene-Conjugated Microporous Polymers for Energy Storage. ACS Appl. Energy Mater. 2022. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Sharma, S.U.; Yang, C.-H.; Samy, M.M.; Mohammed, A.A.K.; Chaganti, S.V.; Lee, J.-T.; Wei-Kuo, S. Anthraquinone-Enriched Conjugated Microporous Polymers as Organic Cathode Materials for High-Performance Lithium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 14628–14639. [Google Scholar] [CrossRef]
- Zhang, C.; Qiao, Y.; Xiong, P.; Ma, W.; Bai, P.; Wang, X.; Li, Q.; Zhao, J.; Xu, Y.; Chen, Y.; et al. Conjugated Microporous Polymers with Tunable Electronic Structure for High-Performance Potassium-Ion Batteries. ACS Nano 2019, 13, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wayment, L.J.; Haslam, C.; Yang, X.; Lee, S.-h.; Jin, Y.; Zhang, W. Covalent organic framework based lithium-ion battery: Fundamental, design and characterization. EnergyChem 2021, 3, 100048. [Google Scholar] [CrossRef]
- Huang, N.; Wang, P.; Jiang, D. Covalent organic frameworks: A materials platform for structural and functional designs. Nat. Rev. Mater. 2016, 1, 16068. [Google Scholar] [CrossRef]
- Amin, K.; Ashraf, N.; Mao, L.; Faul, C.F.J.; Wei, Z. Conjugated microporous polymers for energy storage: Recent progress and challenges. Nano Energy 2021, 85, 105958. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, G.; Wang, M.; Li, J.; Lu, T.; Pan, L. Covalent-organic-frameworks derived N-doped porous carbon materials as anode for superior long-life cycling lithium and sodium ion batteries. Carbon 2017, 116, 686–694. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, H.; Wang, X.; Zhu, Z.; Liang, W.; Li, A.; Wen, S.; Deng, W. Conjugated Microporous Polymer-Derived Porous Hard Carbon as High-Rate Long-Life Anode Materials for Lithium Ion Batteries. Energy Technol. 2013, 1, 721–725. [Google Scholar] [CrossRef]
- Ibrahim, M.; Abdelhamid, H.N.; Abuelftooh, A.M.; Mohamed, S.G.; Wen, Z.; Sun, X. Covalent organic frameworks (COFs)-derived nitrogen-doped carbon/reduced graphene oxide nanocomposite as electrodes materials for supercapacitors. J. Energy Storage 2022, 55, 105375. [Google Scholar] [CrossRef]
- Yuan, K.; Hu, T.; Xu, Y.; Graf, R.; Shi, L.; Forster, M.; Pichler, T.; Riedl, T.; Chen, Y.; Scherf, U. Nitrogen-doped porous carbon/graphene nanosheets derived from two-dimensional conjugated microporous polymer sandwiches with promising capacitive performance. Mater. Chem. Front. 2017, 1, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-Based Supercapacitors Produced by Activation of Graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef]
- Jang, B.Z.; Liu, C.; Neff, D.; Yu, Z.; Wang, M.C.; Xiong, W.; Zhamu, A. Graphene Surface-Enabled Lithium Ion-Exchanging Cells: Next-Generation High-Power Energy Storage Devices. Nano Lett. 2011, 11, 3785–3791. [Google Scholar] [CrossRef] [PubMed]
- Sui, D.; Wu, M.; Shi, K.; Li, C.; Lang, J.; Yang, Y.; Zhang, X.; Yan, X.; Chen, Y. Recent progress of cathode materials for aqueous zinc-ion capacitors: Carbon-based materials and beyond. Carbon 2021, 185, 126–151. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, Y. Three-dimensional graphene networks: Synthesis, properties and applications. Natl. Sci. Rev. 2014, 2, 40–53. [Google Scholar] [CrossRef] [Green Version]
- Mao, S.; Lu, G.; Chen, J. Three-dimensional graphene-based composites for energy applications. Nanoscale 2015, 7, 6924–6943. [Google Scholar] [CrossRef] [Green Version]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of Solution-Processed Reduced Graphene Oxide Films as Transparent Conductors. ACS Nano 2008, 2, 463–470. [Google Scholar] [CrossRef]
- Moreno-Fernández, G.; Granados-Moreno, M.; Gómez-Urbano, J.L.; Carriazo, D. Phosphorus-Functionalized Graphene for Lithium-Ion Capacitors with Improved Power and Cyclability. Batter. Supercaps 2021, 4, 469–478. [Google Scholar] [CrossRef]
- Aravindan, V.; Mhamane, D.; Ling, W.C.; Ogale, S.; Madhavi, S. Nonaqueous Lithium-Ion Capacitors with High Energy Densities using Trigol-Reduced Graphene Oxide Nanosheets as Cathode-Active Material. ChemSusChem 2013, 6, 2240–2244. [Google Scholar] [CrossRef]
- Wang, H.; Guan, C.; Wang, X.; Fan, H.J. A High Energy and Power Li-Ion Capacitor Based on a TiO2 Nanobelt Array Anode and a Graphene Hydrogel Cathode. Small 2015, 11, 1470–1477. [Google Scholar] [CrossRef]
- Li, H.; Shen, L.; Wang, J.; Fang, S.; Zhang, Y.; Dou, H.; Zhang, X. Three-dimensionally ordered porous TiNb2O7 nanotubes: A superior anode material for next generation hybrid supercapacitors. J. Mater. Chem. A 2015, 3, 16785–16790. [Google Scholar] [CrossRef]
- Ye, L.; Liang, Q.; Lei, Y.; Yu, X.; Han, C.; Shen, W.; Huang, Z.-H.; Kang, F.; Yang, Q.-H. A high performance Li-ion capacitor constructed with Li4Ti5O12/C hybrid and porous graphene macroform. J. Power Sources 2015, 282, 174–178. [Google Scholar] [CrossRef]
- Sui, D.; Xu, L.; Zhang, H.; Sun, Z.; Kan, B.; Ma, Y.; Chen, Y. A 3D cross-linked graphene-based honeycomb carbon composite with excellent confinement effect of organic cathode material for lithium-ion batteries. Carbon 2020, 157, 656–662. [Google Scholar] [CrossRef]
- Stoller, M.D.; Murali, S.; Quarles, N.; Zhu, Y.; Potts, J.R.; Zhu, X.; Ha, H.-W.; Ruoff, R.S. Activated graphene as a cathode material for Li-ion hybrid supercapacitors. Phys. Chem. Chem. Phys. 2012, 14, 3388–3391. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, Q.; Ding, B.; Wang, J.; Malgras, V.; Jiang, J.; Li, Z.; Chen, S.; Dou, H.; Alshehri, S.M.; et al. Lithium-ion capacitor based on nanoarchitectured polydopamine/graphene composite anode and porous graphene cathode. Carbon 2020, 167, 627–633. [Google Scholar] [CrossRef]
- Ma, X.; Gao, D. High Capacitive Storage Performance of Sulfur and Nitrogen Codoped Mesoporous Graphene. ChemSusChem 2018, 11, 1048–1055. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, L.; Song, X.; Yu, Z.; Zhao, L.; Yu, Y.; Xiao, Z.; Ning, G.; Gao, J. Superior capacitive behaviors of the micron-sized porous graphene belts with high ratio of length to diameter. Carbon 2018, 140, 314–323. [Google Scholar] [CrossRef]
- Jin, L.; Guo, X.; Gong, R.; Zheng, J.; Xiang, Z.; Zhang, C.; Zheng, J.P. Target-oriented electrode constructions toward ultra-fast and ultra-stable all-graphene lithium ion capacitors. Energy Storage Mater. 2019, 23, 409–417. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, Z.; Zhang, T.; Qin, B.; Sui, D.; Xie, Y.; Ma, Y.; Chen, Y. Porous asphalt/graphene composite for supercapacitors with high energy density at superior power density without added conducting materials. J. Mater. Chem. A 2017, 5, 21757–21764. [Google Scholar] [CrossRef]
- Sui, D.; Wu, M.; Liu, Y.; Yang, Y.; Zhang, H.; Ma, Y.; Zhang, L.; Chen, Y. High performance Li-ion capacitor fabricated with dual graphene-based materials. Nanotechnology 2020, 32, 015403. [Google Scholar] [CrossRef]
- Yu, Y.; Cui, C.; Qian, W.; Xie, Q.; Zheng, C.; Kong, C.; Wei, F. Carbon nanotube production and application in energy storage. Asia-Pac. J. Chem. Eng. 2013, 8, 234–245. [Google Scholar] [CrossRef]
- Frackowiak, E.; Béguin, F. Electrochemical storage of energy in carbon nanotubes and nanostructured carbons. Carbon 2002, 40, 1775–1787. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, T.; Li, L.; Peng, H. Carbon Nanotubes for Flexible Fiber Batteries. In Nanoporous Carbons for Soft and Flexible Energy Devices; Borghi, F., Soavi, F., Milani, P., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–22. [Google Scholar] [CrossRef]
- Wen, L.; Li, F.; Cheng, H.-M. Carbon Nanotubes and Graphene for Flexible Electrochemical Energy Storage: From Materials to Devices. Adv. Mater. 2016, 28, 4306–4337. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Huang, Y.; Zhang, F.; Yang, X.; Ma, Y.; Chen, Y. Preventing Graphene Sheets from Restacking for High-Capacitance Performance. J. Phys. Chem. C 2011, 115, 23192–23197. [Google Scholar] [CrossRef]
- Wang, J.-A.; Li, S.-M.; Wang, Y.-S.; Lan, P.-Y.; Liao, W.-H.; Hsiao, S.-T.; Lin, S.-C.; Lin, C.-W.; Ma, C.-C.M.; Hu, C.-C. Preparation and Properties of NrGO-CNT Composite for Lithium-Ion Capacitors. J. Electrochem. Soc. 2017, 164, A3657–A3665. [Google Scholar] [CrossRef]
- Aphirakaramwong, C.; Phattharasupakun, N.; Suktha, P.; Krittayavathananon, A.; Sawangphruk, M. Lightweight Multi-Walled Carbon Nanotube/N-Doped Graphene Aerogel Composite for High-Performance Lithium-Ion Capacitors. J. Electrochem. Soc. 2019, 166, A532–A538. [Google Scholar] [CrossRef]
- Salvatierra, R.V.; Zakhidov, D.; Sha, J.; Kim, N.D.; Lee, S.-K.; Raji, A.-R.O.; Zhao, N.; Tour, J.M. Graphene Carbon Nanotube Carpets Grown Using Binary Catalysts for High-Performance Lithium-Ion Capacitors. ACS Nano 2017, 11, 2724–2733. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y. Capacitive Energy Storage in Nanostructured Carbon–Electrolyte Systems. Acc. Chem. Res. 2013, 46, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Weng, Z.; Li, P.; Tao, Y.; Cui, C.; Zhang, L.; Lin, W.; Gao, Y.; Kong, D.; Yang, Q.-H. Electrode thickness matching for achieving high-volumetric-performance lithium-ion capacitors. Energy Storage Mater. 2019, 18, 133–138. [Google Scholar] [CrossRef]
- Zong, J.; Ni, W.; Xu, H.; Ding, F.; Wang, T.; Feng, W.; Liu, X. High tap-density graphene cathode material for lithium-ion capacitors via a mass-scalable synthesis method. Chem. Eng. J. 2019, 360, 1233–1240. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, J.; Kong, D.; Zhang, C.; Han, D.; Han, J.; Tao, Y.; Lv, W.; Yang, Q.-H. Practical Graphene Technologies for Electrochemical Energy Storage. Adv. Funct. Mater. 2022, 32, 2204272. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, Y.; Zhao, Z.; Wang, Y.; Wu, Y.; Wang, X. Phytic acid assisted formation of P-doped hard carbon anode with enhanced capacity and rate capability for lithium ion capacitors. J. Power Sources 2020, 474, 228500. [Google Scholar] [CrossRef]
- Zeng, D.; Xiong, H.; Qi, K.; Guo, X.; Qiu, Y. Constructing N-doping biomass-derived carbon with hierarchically porous architecture to boost fast reaction kinetics for higfh-performance lithium storage. J. Colloid Interface Sci. 2022, 605, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Z.; Dou, M.; Li, Z.; Wang, F. Nitrogen and oxygen co-doped porous carbon nanosheets as high-rate and long-lifetime anode materials for high-performance Li-ion capacitors. Carbon 2019, 151, 28–35. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, W.; Lyu, Y.; Zhang, Y.; Wang, H.; Liu, Y.; Li, D. All-carbon lithium capacitor based on salt crystal-templated, N-doped porous carbon electrodes with superior energy storage. J. Mater. Chem. A 2018, 6, 18276–18285. [Google Scholar] [CrossRef]
- Sun, F.; Wu, H.B.; Liu, X.; Liu, F.; Han, R.; Qu, Z.; Pi, X.; Wang, L.; Gao, J.; Lu, Y. A high-rate and ultrastable anode enabled by boron-doped nanoporous carbon spheres for high-power and long life lithium ion capacitors. Mater. Today Energy 2018, 9, 428–439. [Google Scholar] [CrossRef]
- Zou, K.; Guan, Z.; Deng, Y.; Chen, G. Nitrogen-rich porous carbon in ultra-high yield derived from activation of biomass waste by a novel eutectic salt for high performance Li-ion capacitors. Carbon 2020, 161, 25–35. [Google Scholar] [CrossRef]
- Luan, Y.; Hu, R.; Fang, Y.; Zhu, K.; Cheng, K.; Yan, J.; Ye, K.; Wang, G.; Cao, D. Nitrogen and Phosphorus Dual-Doped Multilayer Graphene as Universal Anode for Full Carbon-Based Lithium and Potassium Ion Capacitors. Nano-Micro Lett. 2019, 11, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, X.; He, J.; Wang, K.; Li, X.; Wang, X.; Yang, Z.; Wang, N.; Zhang, Y.; Huang, C. Fluorine-Enriched Graphdiyne as an Efficient Anode in Lithium-Ion Capacitors. ChemSusChem 2019, 12, 1342–1348. [Google Scholar] [CrossRef]
- Shen, X.; Yang, Z.; Wang, K.; Wang, N.; He, J.; Du, H.; Huang, C. Nitrogen-Doped Graphdiyne as High-capacity Electrode Materials for Both Lithium-ion and Sodium-ion Capacitors. ChemElectroChem 2018, 5, 1435–1443. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, H.; Li, X.; Wang, Z.; Wang, J.; Wang, Y.; Yan, Z.; Zhang, D. Graphitic carbon balanced between high plateau capacity and high rate capability for lithium ion capacitors. J. Mater. Chem. A 2017, 5, 15302–15309. [Google Scholar] [CrossRef]
- Li, G.; Huang, Y.; Yin, Z.; Guo, H.; Liu, Y.; Cheng, H.; Wu, Y.; Ji, X.; Wang, J. Defective synergy of 2D graphitic carbon nanosheets promotes lithium-ion capacitors performance. Energy Storage Mater. 2020, 24, 304–311. [Google Scholar] [CrossRef]
- Zeng, D.; Xiong, H.; Wu, L.; Zhang, Y.; Qi, K.; Guo, X.; Qiu, Y. High-energy graphite microcrystalline carbon for high-performance lithium-ion capacitor: Diffusion kinetics and lithium-storage mechanism. J. Colloid Interface Sci. 2022, 623, 1190–1199. [Google Scholar] [CrossRef]
- Kumagai, S.; Ishikawa, T.; Sawa, N. Cycle performance of lithium-ion capacitors using graphite negative electrodes at different pre-lithiation levels. J. Energy Storage 2015, 2, 1–7. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, C.; Zhang, J.; Jing, Q.; Ma, B.; Chen, Y.; Zhang, W. Graphite Recycling from the Spent Lithium-Ion Batteries by Sulfuric Acid Curing–Leaching Combined with High-Temperature Calcination. ACS Sustain. Chem. Eng. 2020, 8, 9447–9455. [Google Scholar] [CrossRef]
- Ma, X.; Chen, M.; Chen, B.; Meng, Z.; Wang, Y. High-Performance Graphite Recovered from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 19732–19738. [Google Scholar] [CrossRef]
- Kayakool, F.A.; Gangaja, B.; Nair, S.; Santhanagopalan, D. Li-based all-carbon dual-ion batteries using graphite recycled from spent Li-ion batteries. Sustain. Mater. Technol. 2021, 28, e00262. [Google Scholar] [CrossRef]
- Divya, M.L.; Natarajan, S.; Lee, Y.-S.; Aravindan, V. Achieving high-energy dual carbon Li-ion capacitors with unique low- and high-temperature performance from spent Li-ion batteries. J. Mater. Chem. A 2020, 8, 4950–4959. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, X.; Huang, L.; Chen, J. Strategies for Rational Design of High-Power Lithium-ion Batteries. Energy Environ. Mater. 2021, 4, 19–45. [Google Scholar] [CrossRef]
- Sivakkumar, S.R.; Nerkar, J.Y.; Pandolfo, A.G. Rate capability of graphite materials as negative electrodes in lithium-ion capacitors. Electrochim. Acta 2010, 55, 3330–3335. [Google Scholar] [CrossRef]
- Huang, L.; Li, H.; Wang, X.; Ding, Y.; Wang, J.; Jiang, L. High-efficiency, self-grinding exfoliation of small graphene nanosheets from microcrystalline graphite driven by microbead milling as conductive additives. Sci. China Mater. 2022, 65, 2463–2471. [Google Scholar] [CrossRef]
- Yu, X.; Zhan, C.; Lv, R.; Bai, Y.; Lin, Y.; Huang, Z.-H.; Shen, W.; Qiu, X.; Kang, F. Ultrahigh-rate and high-density lithium-ion capacitors through hybriding nitrogen-enriched hierarchical porous carbon cathode with prelithiated microcrystalline graphite anode. Nano Energy 2015, 15, 43–53. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Xiao, J.; Ren, N.; Pan, B.; Chen, C.-s.; Chen, C.-h. Introducing a Pseudocapacitive Lithium Storage Mechanism into Graphite by Defect Engineering for Fast-Charging Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 16279–16288. [Google Scholar] [CrossRef] [PubMed]
- Pendashteh, A.; Orayech, B.; Suhard, H.; Jauregui, M.; Ajuria, J.; Silván, B.; Clarke, S.; Bonilla, F.; Saurel, D. Boosting the performance of soft carbon negative electrode for high power Na-ion batteries and Li-ion capacitors through a rational strategy of structural and morphological manipulation. Energy Storage Mater. 2022, 46, 417–430. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, X.; Liu, W.; Wang, K.; Li, C.; Li, Z.; Ma, Y. Electrochemical performances and capacity fading behaviors of activated carbon/hard carbon lithium ion capacitor. Electrochim. Acta 2017, 235, 158–166. [Google Scholar] [CrossRef]
- Xie, F.; Xu, Z.; Jensen, A.C.S.; Au, H.; Lu, Y.; Araullo-Peters, V.; Drew, A.J.; Hu, Y.-S.; Titirici, M.-M. Hard–Soft Carbon Composite Anodes with Synergistic Sodium Storage Performance. Adv. Funct. Mater. 2019, 29, 1901072. [Google Scholar] [CrossRef]
- Dou, X.; Hasa, I.; Saurel, D.; Vaalma, C.; Wu, L.; Buchholz, D.; Bresser, D.; Komaba, S.; Passerini, S. Hard carbons for sodium-ion batteries: Structure, analysis, sustainability, and electrochemistry. Mater. Today 2019, 23, 87–104. [Google Scholar] [CrossRef]
- Xie, L.; Tang, C.; Bi, Z.; Song, M.; Fan, Y.; Yan, C.; Li, X.; Su, F.; Zhang, Q.; Chen, C. Hard Carbon Anodes for Next-Generation Li-Ion Batteries: Review and Perspective. Adv. Energy Mater. 2021, 11, 2101650. [Google Scholar] [CrossRef]
- Cao, B.; Liu, H.; Xu, B.; Lei, Y.; Chen, X.; Song, H. Mesoporous soft carbon as an anode material for sodium ion batteries with superior rate and cycling performance. J. Mater. Chem. A 2016, 4, 6472–6478. [Google Scholar] [CrossRef]
- Zhao, L.-F.; Hu, Z.; Lai, W.-H.; Tao, Y.; Peng, J.; Miao, Z.-C.; Wang, Y.-X.; Chou, S.-L.; Liu, H.-K.; Dou, S.-X. Hard Carbon Anodes: Fundamental Understanding and Commercial Perspectives for Na-Ion Batteries beyond Li-Ion and K-Ion Counterparts. Adv. Energy Mater. 2021, 11, 2002704. [Google Scholar] [CrossRef]
- Schroeder, M.; Winter, M.; Passerini, S.; Balducci, A. On the Use of Soft Carbon and Propylene Carbonate-Based Electrolytes in Lithium-Ion Capacitors. J. Electrochem. Soc. 2012, 159, A1240–A1245. [Google Scholar] [CrossRef]
- Schroeder, M.; Winter, M.; Passerini, S.; Balducci, A. On the cycling stability of lithium-ion capacitors containing soft carbon as anodic material. J. Power Sources 2013, 238, 388–394. [Google Scholar] [CrossRef]
- Lim, Y.-G.; Park, M.-S.; Kim, K.J.; Jung, K.-S.; Kim, J.H.; Shahabuddin, M.; Byun, D.; Yu, J.-S. Incorporation of conductive polymer into soft carbon electrodes for lithium ion capacitors. J. Power Sources 2015, 299, 49–56. [Google Scholar] [CrossRef]
- de las Casas, C.; Li, W. A review of application of carbon nanotubes for lithium ion battery anode material. J. Power Sources 2012, 208, 74–85. [Google Scholar] [CrossRef]
- Al Hassan, M.R.; Sen, A.; Zaman, T.; Mostari, M.S. Emergence of graphene as a promising anode material for rechargeable batteries: A review. Mater. Today Chem. 2019, 11, 225–243. [Google Scholar] [CrossRef]
- Yoo, E.; Kim, J.; Hosono, E.; Zhou, H.-s.; Kudo, T.; Honma, I. Large Reversible Li Storage of Graphene Nanosheet Families for Use in Rechargeable Lithium Ion Batteries. Nano Lett. 2008, 8, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Wu, D.; Li, S.; Zhang, F.; Feng, X. Graphene: A Two-Dimensional Platform for Lithium Storage. Small 2013, 9, 1173–1187. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, T.; Zhang, F.; Wang, Y.; Ma, Y.; Huang, Y.; Liu, Y.; Chen, Y. In situ synthesis of graphene/single-walled carbon nanotube hybrid material by arc-discharge and its application in supercapacitors. Nano Energy 2012, 1, 820–827. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, J.; Qin, F.; Yuan, J.; Zhang, K.; Li, J.; Zhu, D.-M.; Qin, L.-C. Hybrid lithium-ion capacitors with asymmetric graphene electrodes. J. Mater. Chem. A 2017, 5, 13601–13609. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Liu, H.; Guo, Y.; Li, Y.; Zhu, D. Architecture of graphdiyne nanoscale films. Chem. Commun. 2010, 46, 3256–3258. [Google Scholar] [CrossRef]
- Gao, X.; Liu, H.; Wang, D.; Zhang, J. Graphdiyne: Synthesis, properties, and applications. Chem. Soc. Rev. 2019, 48, 908–936. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, X.; Yang, N. Graphdiyne Electrochemistry: Progress and Perspectives. Small 2022, 18, 2201135. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Yang, H.; Huang, C.; He, J.; Liu, H.; Li, Y. Graphdiyne applied for lithium-ion capacitors displaying high power and energy densities. Nano Energy 2016, 22, 615–622. [Google Scholar] [CrossRef]











| Carbon Cathodes | Performances of DC-LICs | Ref. | |||||
|---|---|---|---|---|---|---|---|
| Materials | Specific Capacity (mAh g−1) | Rate Capability (mAh g−1) | Voltage (V) | Maximum Energy Density (Wh kg−1) | Maximum Power Density (kW kg−1) | Cycling Stability | |
| Commercial AC (YP-50F) | 48 | 35 @ 1 A g−1 | 2.0−4.0 | 73.0 | / | 85% after 1000 cycles | [50] |
| KHPC−K | 100.5 | 75 @ 10 A g−1 | 0.01–4.0 | 169.0 | 97.0 | 77.7% after 5000 cycles | [51] |
| NHCN-2 | 125 | 98 @ 15 A g−1 | 2.0–4.5 | 146.0 | 52.0 | 91% after 40,000 cycles | [52] |
| LPCs-3 | 80 | 26 @ 10 A g−1 | 2.0–4.0 | 97.0 | 11.4 | 92.3% after 5000 cycles | [53] |
| DPC-MK | 149 | 69 @ 50 A g−1 | 1.0–4.2 | 160.6 | 39.7 | 95.6% after 8000 cycles | [54] |
| 0.1-BNC | 113 | 63 @ 10 A g−1 | 0.02–4.5 | 220.0 | 22.5 | 81% after 5000 cycles | [55] |
| S-NPC-40 | 95.9 | 43.2 @ 10 A g−1 | 0–4.0 | 176.1 | 20.0 | 82% after 20,000 cycles | [56] |
| CHPC | 132 | 100 @ 8 A g−1 | 0.01–4.2 | 220 | 66.9 | 70% after 3000 cycles | [57] |
| N/S-CNF0.25 | 133 | 102 @ 10 A g−1 | 1.0–4.3 | 154.0 | 18.6 | 92% after 6000 cycles | [58] |
| PHNCNB | 72 | 51 @ 10 A g−1 | 1.0–4.0 | 148.5 | 25.0 | 90% after 8000 cycles | [59] |
| NHPCS | 74 | 64 @ 5 A g−1 | 0–4.2 | 151.0 | 10.7 | 96.3% after 3000 cycles | [60] |
| NCNs-2 | 115 | 62 @ 10 A g−1 | 0–4.5 | 218.4 | 22.5 | 84.5% after 10,000 cycles | [61] |
| BNC | 75.2 | 51.4 @ 5 A g−1 | 1.0–4.0 | 115.5 | 10.0 | 71.6% after 2000 cycles | [62] |
| ANCS | 113 | 67 @ 10 A g−1 | 0–4.5 | 206.7 | 22.5 | 86.6% after 10,000 cycles | [63] |
| NPCS-1 | 97.4 | 51.3 @ 10 A g−1 | 0–4.0 | 135.6 | 10.0 | 82% after 10,000 cycles | [64] |
| NPCNF | 122 | 53 @ 100 A g−1 | 1.0–4.3 | 143.0 | 45.0 | 83.1% after 10,000 cycles | [65] |
| URGO | 35 | 29 @ 1.1 A g−1 | 2.0–4.0 | 106.0 | 4.2 | ~100% after 1000 cycles | [66] |
| PRGO | 171 | 92.3 @ 8.71 A g−1 | 0.01–4.0 | 262.0 | 9.0 | 91% after 4000 cycles | [67] |
| NGF-0 | 82 | 61 @ 8 A g−1 | 1.0–4.0 | 147 | 48.9 | 87% after 10,000 cycles | [68] |
| GPC | 95 | 40 @ 5 A g−1 | 0–4.2 | 142.9 | 12.1 | 88% after 5000 cycles | [18] |
| SGCs | 257.1 | 147.7 @ 6 A g−1 | 0–4.0 | 249.9 | 19.62 | 95.4% after 10,000 cycles | [69] |
| A-N-GS | 104 | 57 @ 5 A g−1 | 0–4.5 | 187.9 | 11.25 | 93.5% after 3000 cycles | [70] |
| MRPG/CNT | 108 | 83.3 @ 5 A g− 1 | 0–4.5 | 232.6 | 45.2 | 86% after 5000 cycles | [71] |
| Zn90Co10-APC | 118.8 | 50 @ 5 A g− 1 | 2.0–4.0 | 108 | 15.0 | 86% after 10,000 cycles | [72] |
| Carbon Anodes | Performances of DC-LICs | Ref. | |||||
|---|---|---|---|---|---|---|---|
| Materials | Specific Capacity (mAh g−1) | Rate Capability (mAh g−1) | Voltage (V) | Maximum Energy Density (Wh kg−1) | Maximum Power Density (kW kg−1) | Cycling Stability | |
| HC | 423 | 100 @ 50 C | 1.5−4.2 | 110.0 | 25.0 | 81% after 10,000 cycles | [97] |
| HC-rGO | 450 | 162 @ 10 C | 1.5−4.2 | 130.0 | 5.5 | 88% after 10,000 cycles | [92] |
| PHC-4 | 1040.2 | 231.7 @ 6.4 A g−1 | 2.0−4.0 | 104.0 | 11.9 | 84.7% after 5000 cycles | [142] |
| KHPC-600 | 1064 | 280 @ 10 A g−1 | 0.01−4.0 | 169.0 | 97.0 | 77.7% after 5000 cycles | [51] |
| HNBC | 1392 | 620 @ 1 A g−1 | 0−4.5 | 186.31 | 11.25 | 81.9% after 10,000 cycles | [143] |
| G/SC | 360 | 200 @ 4 A g−1 | 2.0−4.0 | 151.0 | 18.9 | 93.8% after 10,000 cycles | [78] |
| SLC | 829 | 148 @ 10 A g−1 | 0−4.0 | 127.0 | 33.57 | 99% after 100,000 cycles | [91] |
| NOPCNS | 810 | 249 @ 50 A g−1 | 2.0−4.2 | 184.0 | 78.1 | 70% after 10,000 cycles | [144] |
| GOCAF | 398 | 195 @ 10 C | 1.5−4.2 | 100.0 | 9.0 | 80% after 15,000 cycles | [93] |
| NOPCNS | 810 | 249 @ 50 A g−1 | 0−4.0 | 184.0 | 78.1 | 70% after 10,000 cycles | [144] |
| HNBC | 1392 | 300 @ 5 A g−1 | 0−4.5 | 186.31 | 11.25 | 81.9% after 10,000 cycles | [143] |
| NPC | 1740 | 369 @ 10 A g−1 | 0−4.5 | 203 | 90.0 | 80% after 20,000 cycles | [145] |
| HNCNBs | 850 | 321 @ 20 A g−1 | 1.0−4.0 | 148.5 | 25.0 | 90% after 8000 cycles | [59] |
| BDC | 1018 | 564 @ 5 A g−1 | 2.0−4.5 | 207.0 | 17.06 | 88% after 15,000 cycles | [146] |
| NDPC-0.5 | 1000 | 295 @ 5 A g−1 | 0−4.0 | 116.9 | 10.0 | 81% after 8000 cycles | [147] |
| FRGO | 660 | 220 @ 3.72 A g−1 | 0−4.2 | 148.3 | 7.8 | 80% after 3000 cycles | [18] |
| PRGO | 982 | 166 @ 20 A g−1 | 0.01−4.0 | 262.0 | 9.0 | 91% after 4000 cycles | [67] |
| PDA-GN | 1150 | 371 @ 5 A g−1 | 0−4.2 | 135.6 | 21.0 | 65% after 3000 cycles | [124] |
| SHSG | 854 | 333 @ 10 C | 2.0−4.5 | 146.0 | 52.0 | ~91% after 40,000 cycles | [52] |
| NPG | 859 | 758 @ 2 A g−1 | 1.0−4.0 | 195.0 | 14.98 | ~100% after 5000 cycles | [148] |
| G-COOH | 450 | 145 @ 10 A g−1 | 1.0–4.2 | 120.8 | 53.55 | 98.9% after 50,000 cycles | [127] |
| rGO800-P | 461 | 185 @ 10 C | 1.5−4.5 | 91.0 | 26.0 | 76% after 10,000 cycles | [117] |
| F-GDY | 1825.9 | 979.2 @ 5 A g−1 | 2.0−4.0 | 200.2 | 13.117 | 80% after 6000 cycles | [149] |
| N-GDY | 1096.1 | 440 @ 4 A g−1 | 2.0−4.0 | 174.0 | 11.25 | 89.7% after 2000 cycles | [150] |
| GC1100 | 354 | 222 @ 2 A g−1 | 2.0−4.0 | 104.0 | 6.628 | 96.5 % after 3000 cycles | [151] |
| GNS-13 | 356 | 66.7 @ 5 A g−1 | 2.0−4.0 | 112.0 | 19.6 | 96.5% after 5000 cycles | [152] |
| GMC | 119 | 378 @ 1 A g−1 | 0−4.5 | 190.63 | 11.25 | 81.8% after 10,000 cycles | [153] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Y.; Li, C.; Ye, Z.; Li, H.; Yang, Y.; Sui, D.; Lu, Y. Advances of Carbon Materials for Dual-Carbon Lithium-Ion Capacitors: A Review. Nanomaterials 2022, 12, 3954. https://doi.org/10.3390/nano12223954
Duan Y, Li C, Ye Z, Li H, Yang Y, Sui D, Lu Y. Advances of Carbon Materials for Dual-Carbon Lithium-Ion Capacitors: A Review. Nanomaterials. 2022; 12(22):3954. https://doi.org/10.3390/nano12223954
Chicago/Turabian StyleDuan, Ying, Changle Li, Zhantong Ye, Hongpeng Li, Yanliang Yang, Dong Sui, and Yanhong Lu. 2022. "Advances of Carbon Materials for Dual-Carbon Lithium-Ion Capacitors: A Review" Nanomaterials 12, no. 22: 3954. https://doi.org/10.3390/nano12223954