Mesoporous Silica and Oligo (Ethylene Glycol) Methacrylates-Based Dual-Responsive Hybrid Nanogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Cells
2.2. Methods
2.2.1. Synthesis of Mesoporous Silica Nanoparticles
2.2.2. Surface Modification of Mesoporous Silica Nanoparticles
2.2.3. Synthesis of Hybrid Nanogels
2.2.4. Characterization of Hybrid Nanogels
2.2.5. Thermo-Responsive Behavior
2.2.6. pH-Responsiveness
2.2.7. Drug Encapsulation
2.2.8. Drug Release
2.2.9. Cell Viability Assay
3. Results and Discussion
3.1. Synthesis and Structural Aspects of Hybrid Nanogels
3.2. Characterization of Hydrodynamic Diameters and Dual Smart Responsiveness
3.2.1. Effect of Molar Ratio of OEG Monomers and Crosslinking
3.2.2. Effect of Incorporation of AA and IA
3.2.3. Effect of Increasing IA Concentration
3.2.4. Characterization of the pH-Responsiveness
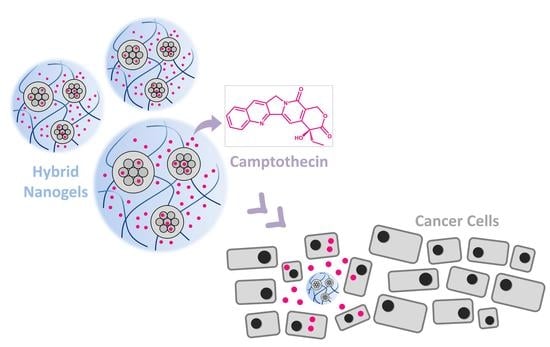
3.3. Further Characterization of the Selected Hybrid Nanogel, Camptothecin Encapsulation and Release Studies
- Encapsulation Efficiency = (94 ± 2)%
- Drug loading content = (52.0 ± 0.7)%
3.4. Cytotoxicity Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quesada-González, D.; Merkoçi, A. Nanomaterial-based devices for point-of-care diagnostic applications. Chem. Soc. Rev. 2018, 47, 4697–4709. [Google Scholar] [CrossRef] [PubMed]
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Ji, Q.; Nakanishi, W.; Hill, J.P.; Aono, M. Nanoarchitectonics: A new materials horizon for nanotechnology. Mater. Horiz. 2015, 2, 406–413. [Google Scholar] [CrossRef]
- Macchione, M.A.; Biglione, C.; Strumia, M. Design, Synthesis and Architectures of Hybrid Nanomaterials for Therapy and Diagnosis Applications. Polymers 2018, 10, 527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Lu, K.J.; Yu, C.H.; Huang, Q.L.; Du, Y.Z. Nano-drug delivery systems in wound treatment and skin regeneration. J. Nanobiotechnol. 2019, 17, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Simeonov, M.; Gussiyska, A.; Mironova, J.; Nikolova, D.; Apostolov, A.; Sezanova, K.; Dyulgerova, E.; Vassileva, E. Novel hybrid chitosan/calcium phosphates microgels for remineralization of demineralized enamel—A model study. Eur. Polym. J. 2019, 119, 14–21. [Google Scholar] [CrossRef]
- Cheng, C.A.; Deng, T.; Lin, F.C.; Cai, Y.; Zink, J.I. Supramolecular nanomachines as stimuli-responsive gatekeepers on mesoporous silica nanoparticles for antibiotic and cancer drug delivery. Theranostics 2019, 9, 3341–3364. [Google Scholar] [CrossRef]
- Zhao, N.; Yan, L.; Zhao, X.; Chen, X.; Li, A.; Zheng, D.; Zhou, X.; Dai, X.; Xu, F.J. Versatile Types of Organic/Inorganic Nanohybrids: From Strategic Design to Biomedical Applications. Chem. Rev. 2019, 119, 1666–1762. [Google Scholar] [CrossRef]
- Murar, M.; Albertazzi, L.; Pujals, S. Advanced Optical Imaging-Guided Nanotheranostics towards Personalized Cancer Drug Delivery. Nanomaterials 2022, 12, 399. [Google Scholar] [CrossRef]
- Meroni, D.; Ardizzone, S. Preparation and application of hybrid nanomaterials. Nanomaterials 2018, 8, 891. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous materials for drug delivery. Angew. Chemie Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef] [PubMed]
- Maggini, L.; Cabrera, I.; Ruiz-Carretero, A.; Prasetyanto, E.A.; Robinet, E.; de Cola, L. Breakable mesoporous silica nanoparticles for targeted drug delivery. Nanoscale 2016, 8, 7240–7247. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Botella, P.; Rivero-Buceta, E. Silica-Based Stimuli-Responsive Systems for Antitumor Drug Delivery and Controlled Release. Pharmaceutics 2022, 14, 110. [Google Scholar] [CrossRef]
- Rosenholm, J.M.; Mamaeva, V.; Sahlgren, C.; Lindén, M. Nanoparticles in Targeted Cancer Therapy Mesoporous Silica Nanoparticles Entering Preclinical Development Stage. Nanomedicine 2012, 7, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Singh, R.K.; Khanal, D.; Patel, K.D.; Lee, E.J.; Leong, K.W.; Chrzanowski, W.; Kim, H.W. Smart multifunctional drug delivery towards anticancer therapy harmonized in mesoporous nanoparticles. Nanoscale 2015, 7, 14191–14216. [Google Scholar] [CrossRef]
- Yuan, L.; Tang, Q.; Yang, D.; Zhang, J.Z.; Zhang, F.; Hu, J. Preparation of pH-Responsive Mesoporous Silica Nanoparticles and Their Application in Controlled Drug Delivery. J. Phys. Chem. C 2011, 115, 9926–9932. [Google Scholar] [CrossRef]
- Gisbert-Garzarán, M.; Vallet-Regí, M. Redox-responsive mesoporous silica nanoparticles for cancer treatment: Recent updates. Nanomaterials 2021, 11, 2222. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Drug Delivery. Adv. Funct. Mater. 2020, 30, 1902634. [Google Scholar] [CrossRef]
- Aguilar, M.R.; Román, J.S. Smart Polymers and Their Applications; Elsevier: Oxford, UK, 2019. [Google Scholar]
- Yan, Q.; Guo, X.; Huang, X.; Meng, X.; Liu, F.; Dai, P.; Wang, Z.; Zhao, Y. Gated Mesoporous Silica Nanocarriers for Hypoxia-Responsive Cargo Release. ACS Appl. Mater. Interfaces 2019, 11, 24377–24385. [Google Scholar] [CrossRef]
- Castillo-Henríquez, L.; Castro-Alpízar, J.; Lopretti-Correa, M.; Vega-Baudrit, J. Exploration of bioengineered scaffolds composed of thermo-responsive polymers for drug delivery in wound healing. Int. J. Mol. Sci. 2021, 22, 1408. [Google Scholar] [CrossRef]
- Abuwatfa, W.A.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. Thermosensitive Polymers and Thermo-Responsive Liposomal Drug Delivery Systems. Polymers 2022, 14, 925. [Google Scholar] [CrossRef] [PubMed]
- Richtering, W.; Alberg, I.; Zentel, R. Nanoparticles in the Biological Context: Surface Morphology and Protein Corona Formation. Small 2020, 16, 2002162–2002170. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Wu, P. Microgels with Linear Thermosensitivity in a Wide Temperature Range. Macromolecules 2016, 49, 6095. [Google Scholar] [CrossRef]
- Du, Z.; Sun, X.; Tai, X.; Wang, G.; Liu, X. Optimizing conditions of preparation of thermoresponsive SiO2-POEGMA particles via AGET-ATRP. Appl. Surf. Sci. 2015, 329, 234–239. [Google Scholar] [CrossRef]
- Constantin, M.; Cristea, M.; Ascenzi, P.; Fundueanu, G. Lower critical solution temperature versus volume phase transition temperature in thermoresponsive drug delivery systems. Express Polym. Lett. 2011, 5, 839–848. [Google Scholar] [CrossRef]
- Vancoillie, G.; Frank, D.; Hoogenboom, R. Thermoresponsive poly (oligo ethylene glycol acrylates). Prog. Polym. Sci. 2014, 39, 1074. [Google Scholar] [CrossRef]
- Lutz, J.F. Polymerization of oligo(ethylene glycol) (meth)acrylates: Toward new generations of smart biocompatible materials. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3459. [Google Scholar] [CrossRef]
- Conzatti, G.; Cavalie, S.; Combes, C.; Torrisani, J.; Carrere, N.; Tourrette, A. Biointerfaces PNIPAM grafted surfaces through ATRP and RAFT polymerization: Chemistry and bioadhesion. Colloids Surf. B Biointerfaces 2017, 151, 143–155. [Google Scholar] [CrossRef] [Green Version]
- Hajebi, S.; Abdollahi, A.; Roghani-Mamaqani, H.; Salami-Kalajahi, M. Hybrid and hollow Poly (N,N-dimethylaminoethyl methacrylate) nanogels as stimuli-responsive carriers for controlled release of doxorubicin. Polymer 2019, 180, 121716. [Google Scholar] [CrossRef]
- Biglione, C.; Sousa-Herves, A.; Menger, M.; Wedepohl, S.; Calderón, M.; Strumia, M.C. Facile ultrasonication approach for the efficient synthesis of ethylene glycol-based thermoresponsive nanogels. RSC Adv. 2015, 5, 15407. [Google Scholar] [CrossRef]
- Cai, Q.; Luo, Z.S.; Pang, W.Q.; Fan, Y.W.; Chen, X.H.; Cui, F.Z. Dilute solution routes to various controllable morphologies of MCM-41 silica with a basic medium. Chem. Mater. 2001, 13, 258–263. [Google Scholar] [CrossRef]
- Rivero-Buceta, E.; Vidaurre-Agut, C.; Vera-Donoso, C.D.; Benlloch, J.M.; Moreno-Manzano, V.; Botella, P. PSMA-Targeted Mesoporous Silica Nanoparticles for Selective Intracellular Delivery of Docetaxel in Prostate Cancer Cells. ACS Omega 2019, 4, 1281–1291. [Google Scholar] [CrossRef] [Green Version]
- Macchione, M.A.; Sacarelli, M.F.; Racca, A.C.; Biglione, C.; Panzetta-Dutari, G.M.; Strumia, M.C. Dual-responsive nanogels based on oligo(ethylene glycol) methacrylates and acidic co-monomers. Soft Matter 2019, 15, 9700–9709. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Sayari, A. Adsorption study of surface and structural properties of MCM-41 materials of different pore sizes. J. Phys. Chem. B 1997, 101, 583–589. [Google Scholar] [CrossRef]
- Llinàs, M.C.; Martínez-Edo, G.; Cascante, A.; Porcar, I.; Borrós, S.; Sánchez-García, D. Preparation of a mesoporous silica-based nano-vehicle for dual DOX/CPT ph-triggered delivery. Drug Deliv. 2019, 25, 1137–1146. [Google Scholar] [CrossRef]
- Duan, X.; Yang, X.; Dai, C.; Tong, T.; Miao, C.; Zheng, J. One-pot synthesis of camptothecin-loaded glutathione-responsive peglyation nanogels as novel antitumor therapeutics. Mater. Express 2019, 9, 757–763. [Google Scholar] [CrossRef]
- Babaei, M.; Abnous, K.; Taghdisi, S.M.; Taghavi, S.; Saljooghi, A.S.; Ramezani, M.; Alibolandi, M. Targeted rod-shaped mesoporous silica nanoparticles for the co-delivery of camptothecin and survivin shRNA in to colon adenocarcinoma in vitro and in vivo. Eur. J. Pharm. Biopharm. 2020, 156, 84–96. [Google Scholar] [CrossRef]
- Enache, D.F.; Vasile, E.; Simonescu, C.M.; Culita, D.; Vasile, E.; Oprea, O.; Pandele, A.M.; Razvan, A.; Dumitru, F.; Nechifor, G. Schiff base-functionalized mesoporous silicas (MCM-41, HMS) as Pb(II) adsorbents. RSC Adv. 2018, 8, 176–189. [Google Scholar] [CrossRef] [Green Version]
- Lutz, J.F.; Weichenhan, K.; Akdemir, Ö.; Hoth, A. About the phase transitions in aqueous solutions of thermoresponsive copolymers and hydrogels based on 2-(2-methoxyethoxy)ethyl methacrylate and oligo(ethylene glycol) methacrylate. Macromolecules 2007, 40, 2503. [Google Scholar] [CrossRef]
- Biglione, C.; Álvarez, J.B.; Wedepohl, S.; Klemke, B.; Strumia, M.C.; Calderon, M. Revealing the NIR Triggered Chemotherapy Therapeutic Window of Magnetic and Thermoresponsive Nanogels. Nanoscale 2020, 12, 21635–21646. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.; Choi, J. Preparation of a Bioactive Poly(methyl methacrylate)/Silica Nanocomposite. Commun. Am. Ceram. Soc. 2002, 85, 1318–1320. [Google Scholar] [CrossRef]
- Llansola Portoles, M.J.; Rodriguez Nieto, F.; Soria, D.B.; Amalvy, J.I.; Peruzzo, P.J.; Mártire, D.O.; Kotler, M.; Holub, O.; Gonzalez, M.C. Photophysical properties of blue—Emitting silicon nanoparticles. J. Phys. Chem. C. Nanomater Interfaces 2009, 113, 13694–13702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Zeng, D.L.; Wang, L.; Zong, B.; Neoh, K.G.; Kang, E.T. Hairy hybrid nanoparticles of magnetic core, fluorescent silica shell, and functional polymer brushes. Macromolecules 2009, 42, 8561–8565. [Google Scholar] [CrossRef]
- Chen, W.-C.; Lee, S.-J. Synthesis and characterization of poly(methyl methacrylate)-silica hybrid optical thin films. Polym. J. 2000, 32, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Miyamae, T.; Yokoyama, H.; Han, S.; Ishizone, T. Surface characterization of block copolymers with water-soluble block by using sum-frequency generation spectroscopy. e-Journal Surf. Sci. Nanotechnol. 2006, 4, 515–520. [Google Scholar] [CrossRef] [Green Version]
- Bergueiro, J.; Calderón, M. Thermoresponsive nanodevices in biomedical applications. Macromol. Biosci. 2015, 15, 183–199. [Google Scholar] [CrossRef]
- Bedoya, D.A.; Figueroa, F.N.; Macchione, M.A.; Strumia, M.C. Stimuli-Responsive Polymeric Systems for Smart Drug Delivery. In Advanced Biopolymeric Systems for Drug Delivery; Nayak, A.K., Hasnain, M.S., Eds.; Springer: Cham, Switzerland, 2020; pp. 115–134. [Google Scholar]
- Blackburn, W.H.; Lyon, L.A. Size-controlled synthesis of monodisperse core/shell nanogels. Colloid Polym. Sci. 2008, 286, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Füllbrandt, M.; von Klitzing, R.; Schönhals, A. The dielectric signature of poly(N-isopropylacrylamide) microgels at the volume phase transition: Dependence on the crosslinking density. Soft Matter 2013, 9, 4464–4471. [Google Scholar] [CrossRef] [Green Version]
- Agnihotri, P.; Raj, R.; Kumar, D.; Dan, A. Short oligo(ethylene glycol) chain incorporated thermoresponsive microgels: From structural analysis to modulation of solution properties. Soft Matter 2020, 16, 7845–7859. [Google Scholar] [CrossRef]
- Spatarelu, C.P.; Chiriac, A.L.; Cursaru, B.; Iordache, T.V.; Gavrila, A.M.; Cojocaru, C.T.; Botez, R.E.; Trica, B.; Sarbu, A.; Teodorescu, M.; et al. Composite nanogels based on zeolite-poly(Ethylene glycol) diacrylate for controlled drug delivery. Nanomaterials 2020, 10, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stock, S.; Röhl, S.; Mirau, L.; Kraume, M.; von Klitzing, R. Maximum Incorporation of Soft Microgel at Interfaces of Water in Oil Emulsion Droplets Stabilized by Solid Silica Spheres. Nanomaterials 2022, 12, 2649. [Google Scholar] [CrossRef] [PubMed]
- García-García, J.M.; Liras, M.; Quijada-Garrido, I.; Gallardo, A.; París, R. Swelling control in thermo-responsive hydrogels based on 2-(2-methoxyethoxy)ethyl methacrylate by crosslinking and copolymerization with N-isopropylacrylamide. Polym. J. 2011, 43, 887–892. [Google Scholar] [CrossRef] [Green Version]
- Becer, C.R.; Hahn, S.; Fijten, M.W.M.; Thijs, H.M.L.; Hoogenboom, R.; Shubert, U.S. Libraries of Methacrylic Acid and Oligo(ethylene glycol) Methacrylate Copolymers with LCST Behavior. Am. Chem. Soc. Polym. Prepr. Div. Polym. Chem. 2008, 49, 511–512. [Google Scholar] [CrossRef]
- Keerl, M.; Richtering, W. Synergistic depression of volume phase transition temperature in copolymer microgels. Colloid Polym. Sci. 2007, 285, 471–474. [Google Scholar] [CrossRef]
- París, R.; Quijada-Garrido, I. Temperature- and pH-responsive behaviour of poly(2-(2-methoxyethoxy)ethyl methacrylate-co-N,N-dimethylaminoethyl methacrylate) hydrogels. Eur. Polym. J. 2010, 46, 2156–2163. [Google Scholar] [CrossRef]
- Rimondino, G.N.; Miceli, E.; Molina, M.; Wedepohl, S.; Thierbach, S.; Rühl, E.; Strumia, M.; Martinelli, M.; Calderón, M. Rational design of dendritic thermoresponsive nanogels that undergo phase transition under endolysosomal conditions. J. Mater. Chem. B 2017, 5, 866–874. [Google Scholar] [CrossRef]
- Macchione, M.A.; Guerrero-Beltrán, C.; Rosso, A.P.; Euti, E.M.; Martinelli, M.; Strumia, M.C.; Muñoz-Fernández, M.Á. Poly(N-vinylcaprolactam) Nanogels with Antiviral Behavior against HIV-1 Infection. Sci. Rep. 2019, 9, 5732–5742. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Raghunathan, K.; Solhaug, H.; Antony, J.; Stenvik, J.; Nilsen, A.M.; Einarsrud, M.A.; Bandyopadhyay, S. Modulating acrylic acid content of nanogels for drug delivery & biocompatibility studies. J. Colloid Interface Sci. 2022, 607, 76–88. [Google Scholar]
- Eslami, P.; Albino, M.; Scavone, F.; Chiellini, F.; Morelli, A.; Baldi, G.; Cappiello, L.; Doumett, S.; Lorenzi, G.; Ravagli, C.; et al. Smart Magnetic Nanocarriers for Multi-Stimuli On-Demand Drug Delivery. Nanomaterials 2022, 12, 303. [Google Scholar] [CrossRef]
- Li, W.T.; Xue, B.X.; Shi, K.; Qu, Y.; Chu, B.Y.; Qian, Z.Y. Magnetic iron oxide nanoparticles/10-hydroxy camptothecin co-loaded nanogel for enhanced photothermal-chemo therapy. Appl. Mater. Today 2019, 14, 84–95. [Google Scholar] [CrossRef]
- Botella, P.; Rivero-Buceta, E. Safe approaches for camptothecin delivery: Structural analogues and nanomedicines. J. Control. Release 2017, 247, 28–54. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, K.; Guo, S.; Duan, X. Nanostructural drug-inorganic clay composites: Structure, thermal property and in vitro release of captopril-intercalated Mg-Al-layered double hydroxides. J. Solid State Chem. 2006, 179, 1792–1801. [Google Scholar] [CrossRef]
- Lim, E.-B.; Vy, T.A.; Lee, S.-W. Comparative release kinetics of small drugs (ibuprofen and acetaminophen) from multifunctional mesoporous silica nanoparticles. J. Mater. Chem. B 2020, 8, 2096–2106. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Jin, L.; Han, J.; Wei, M.; Li, C. Synthesis and controlled release properties of prednisone intercalated mg-al layered double hydroxide composite. Ind. Eng. Chem. Res. 2009, 48, 5590–5597. [Google Scholar] [CrossRef]
- Baltazar, G.C.; Guha, S.; Lu, W.; Lim, J.; Boesze-Battaglia, K.; Laties, A.M.; Tyagi, P.; Kompella, U.B.; Mitchell, C.H. Acidic Nanoparticles Are Trafficked to Lysosomes and Restore an Acidic Lysosomal pH and Degradative Function to Compromised ARPE-19 Cells. PLoS ONE 2012, 7, e49635–e49645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaksonen, M.; Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. Ultrasound responsive mesoporous silica nanoparticles for biomedical applications. Chem. Commun. 2019, 55, 2731–2740. [Google Scholar] [CrossRef] [Green Version]
- Kallury, K.M.R.; Macdonald, P.M.; Thompson, M. Effect of Surface Water and Base Catalysis on the Silanization of Silica by (Aminopropyl) alkoxysilanes Studied by X-ray Photoelectron Spectroscopy and C-13 Cross-Polarization Magic-Angle-Spinning Nuclear- Magnetic-Resonance. Langmuir 1994, 10, 492–499. [Google Scholar] [CrossRef]
- Suri, C.R.; Mishra, G.C. Activating piezoelectric crystal surface by silanization for microgravimetric immunobiosensor application. Biosens. Bioelectron 1996, 11, 1199–1205. [Google Scholar] [CrossRef]
- Bressy, C.; Ngo, V.G.; Ziarelli, F.; Margaillan, A. New insights into the adsorption of 3-(trimethoxysilyl)propylmethacrylate on hydroxylated ZnO nanopowders. Langmuir 2012, 28, 3290–3297. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Matyjaszewski, K. Thermally responsive P(M(EO)2MA-co-OEOMA) copolymers via AGET ATRP in miniemulsion. Macromolecules 2010, 43, 4623–4628. [Google Scholar] [CrossRef]







| Samples | DEGMA:OEGMA (mmol) | MSN@MEMO (mg) | TEGDMA (%) | AA/IA (%) |
|---|---|---|---|---|
| HNG-P(DEGMA-co-OEGMA)1.5 | DEGMA:OEGMA (0.8:0.2) | 0.25 | 1.5 | - |
| HNG-P(DEGMA-co-OEGMA) | DEGMA:OEGMA (0.8:0.2) | 0.25 | - | - |
| HNG-P(DEGMA) | DEGMA (1) | 0.25 | - | |
| HNG-P(DEGMA-co-AA4) | DEGMA (1) | 0.25 | - | 4% AA |
| HNG-P(DEGMA-co-IA4) | DEGMA (1) | 0.25 | - | 4% IA |
| HNG-P(DEGMA-co-IA8) | DEGMA (1) | 0.25 | - | 8% IA |
| HNG-P(DEGMA-co-IA12) | DEGMA (1) | 0.25 | - | 12% IA |
| 8Samples | DEGMA: OEGMA (%) | TEGDMA (%) | AA (%) | Dh ± SD (nm) 25 °C | PDI 25 °C | VPTT (°C) |
|---|---|---|---|---|---|---|
| HNG-P(DEGMA-co-OEGMA)1.5 | 80:20 | 1.5 | - | 222.8 ± 2.8 | 0.202 | 59.5 ± 0.1 |
| HNG-P(DEGMA-co-OEGMA) | 80:20 | - | - | 177.8 ± 4.9 | 0.479 | 46.6 ± 0.3 |
| Samples | Acidic Co-Monomer (%) | Dh ± SD (nm) 25 °C | PDI 25 °C | TCP (°C) |
|---|---|---|---|---|
| HNG-P(DEGMA) | - | 206.9 ± 103.6 | 0.142 | 23.6 ± 0.1 |
| HNG-P(DEGMA-co-AA4) | 4% AA | 237.4 ± 27.7 | 0.210 | 34.6 ± 0.4 |
| HNG-P(DEGMA-co-IA4) | 4% IA | 137.5 ± 40.3 | 0.186 | 28.00 ± 0.02 |
| Samples | Dh ± SD (nm) 25 °C | PDI 25 °C | ζ (mV) | Dh ± SD (nm) 50 °C | PDI 50 °C | TCP (°C) |
|---|---|---|---|---|---|---|
| HNG-P(DEGMA) | 206.9 ± 103.6 | 0.142 | −1.5 | 600.2 ± 26.5 | 0.138 | 23.6 ± 0.1 |
| HNG-P(DEGMA-co-IA4) | 137.5 ± 40.3 | 0.186 | −22.5 | 155.1 ± 6.5 | 0.048 | 28.00 ± 0.02 |
| HNG-P(DEGMA-co-IA8) | 238.1 ± 29.5 | 0.200 | −28.5 | 179.7 ± 60.0 | 0.242 | 29.9 ± 0.2 |
| HNG-P(DEGMA-co-IA12) | 343.2 ± 56.4 | 0.388 | −30.7 | 130.5 ± 11.1 | 0.260 | 37.2 ± 0.3 |
| Cell Lines | CPT | CPT Loaded HNG-P(DEGMA-co-IA12) |
|---|---|---|
| LNCaP | 0.0670 ± 0.0101 | 0.0618 ± 0.0060 |
| NIH 3T3 | 0.0476 ± 0.0039 | 0.0839 ± 0.0069 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macchione, M.A.; Bedoya, D.A.; Rivero-Buceta, E.; Botella, P.; Strumia, M.C. Mesoporous Silica and Oligo (Ethylene Glycol) Methacrylates-Based Dual-Responsive Hybrid Nanogels. Nanomaterials 2022, 12, 3835. https://doi.org/10.3390/nano12213835
Macchione MA, Bedoya DA, Rivero-Buceta E, Botella P, Strumia MC. Mesoporous Silica and Oligo (Ethylene Glycol) Methacrylates-Based Dual-Responsive Hybrid Nanogels. Nanomaterials. 2022; 12(21):3835. https://doi.org/10.3390/nano12213835
Chicago/Turabian StyleMacchione, Micaela A., Dariana Aristizábal Bedoya, Eva Rivero-Buceta, Pablo Botella, and Miriam C. Strumia. 2022. "Mesoporous Silica and Oligo (Ethylene Glycol) Methacrylates-Based Dual-Responsive Hybrid Nanogels" Nanomaterials 12, no. 21: 3835. https://doi.org/10.3390/nano12213835