Molecular Dynamics Simulation on Behaviors of Water Nanodroplets Impinging on Moving Surfaces
Abstract
:1. Introduction
2. Simulation Model and Methodology
3. Results and Discussion
3.1. Calculation of the Contact Angle and Verification of Water Nanodroplet Size
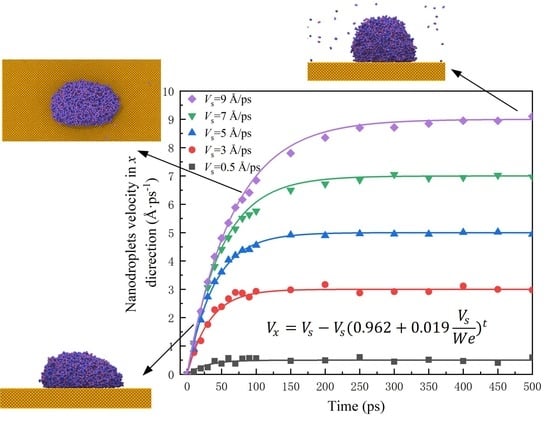
3.2. Effects of Translation Velocity When Water Nanodroplets Impinge on Translation Surfaces
3.3. Effects of the Weber Number When Water Nanodroplets Impinge on Translation Surfaces
3.4. Effects of Vibration Amplitudes on Dynamical Behaviors When Water Nanodroplets Impinge on Vibration Surfaces
3.5. Effects of Vibration Periods on Dynamical Behaviors When Water Nanodroplets Impinge on Vibration Surfaces
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorr, G.J.; Wang, S.; Mayo, L.C.; McCue, S.W.; Forster, W.A.; Hanan, J.; He, X. Impaction of spray droplets on leaves: Influence of formulation and leaf character on shatter, bounce and adhesion. Exp. Fluids 2015, 56, 143. [Google Scholar] [CrossRef] [Green Version]
- Roisman, I.V.; Breitenbach, J.; Tropea, C. Thermal atomisation of a liquid drop after impact onto a hot substrate. J. Fluid Mech. 2018, 842, 87–101. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, T.; He, J. Inkjet Printing Enabled Controllable Paper Superhydrophobization and Its Applications. ACS Appl. Mater. Interfaces 2018, 10, 11343–11349. [Google Scholar] [CrossRef]
- Shi, S.; Lv, C.; Zheng, Q. Drop Impact on Two-Tier Monostable Superrepellent Surfaces. ACS Appl. Mater. Interfaces 2019, 11, 43698–43707. [Google Scholar] [CrossRef]
- Pan, S.; Wang, N.; Xiong, D.; Deng, Y.; Shi, Y. Fabrication of superhydrophobic coating via spraying method and its applications in anti-icing and anti-corrosion. Appl. Surf. Sci. 2016, 389, 547–553. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Wu, X.; Min, J. Impacting-freezing dynamics of a supercooled water droplet on a cold surface: Rebound and adhesion. Int. J. Heat Mass Transf. 2020, 158, 119997. [Google Scholar] [CrossRef]
- Xu, W.; Zheng, H.; Liu, Y.; Zhou, X.; Zhang, C.; Song, Y.; Deng, X.; Leung, M.; Yang, Z.; Xu, R.X.; et al. A droplet-based electricity generator with high instantaneous power density. Nature 2020, 578, 392–396. [Google Scholar] [CrossRef]
- Lee, D.J.; Kim, H.M.; Song, Y.S.; Youn, J.R. Water Droplet Bouncing and Superhydrophobicity Induced by Multiscale Hierarchical Nanostructures. ACS Nano 2012, 6, 7656–7664. [Google Scholar] [CrossRef]
- Shi, Q.M.; Jia, Z.H.; Lin, Q.Y. Dynamic behavior of droplets impacting on microstructured hydrophobic surfaces. Chem. Ind. Eng. Prog. 2016, 35, 3818–3824. [Google Scholar] [CrossRef]
- Jia-Lue, P.; Hao, G.; Tian-Ya, Y.; Xian-Bing, J.; Jin-Liang, X. Behavioral characteristics of droplet collision on Janus particle spheres. Acta Phys. Sin.-Chin. Ed. 2021, 70, 044701. [Google Scholar] [CrossRef]
- Wang, L.-Z.; Zhou, A.; Zhou, J.-Z.; Chen, L.; Yu, Y.-S. Droplet impact on pillar-arrayed non-wetting surfaces. Soft Matter 2021, 17, 5932–5940. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, X.; Gao, S.; Yuan, Z.; Lin, Y.; Chu, F.; Wu, X. Axial spreading of droplet impact on ridged superhydrophobic surfaces. J. Colloid Interface Sci. 2021, 599, 130–139. [Google Scholar] [CrossRef]
- Castrejón-Pita, J.R.; Muñoz-Sánchez, B.N.; Hutchings, I.M.; Castrejón-Pita, A.A. Droplet impact onto moving liquids. J. Fluid Mech. 2016, 809, 716–725. [Google Scholar] [CrossRef] [Green Version]
- Almohammadi, H.; Amirfazli, A. Asymmetric Spreading of a Drop upon Impact onto a Surface. Langmuir 2017, 33, 5957–5964. [Google Scholar] [CrossRef]
- Xie, F.; Lv, S.; Yang, Y.; Wang, X. Contact Time of a Bouncing Nanodroplet. J. Phys. Chem. Lett. 2020, 11, 2818–2823. [Google Scholar] [CrossRef]
- Hubao, A.; Yang, Z.; Hu, R.; Chen, Y.-F.; Yang, L. Effect of Solid–Liquid Interactions on Substrate Wettability and Dynamic Spreading of Nanodroplets: A Molecular Dynamics Study. J. Phys. Chem. C 2020, 124, 23260–23269. [Google Scholar] [CrossRef]
- Liu, H.; Chu, F.; Zhang, J.; Wen, D. Nanodroplets impact on surfaces decorated with ridges. Phys. Rev. Fluids 2020, 5, 074201. [Google Scholar] [CrossRef]
- Kumar, V.A.; Sajith, V.; Sathian, S.P. Influence of Nanoparticles on the Evaporation of a Nanodroplet from Solid Substrates: An Experimental and Molecular Dynamics Investigation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126227. [Google Scholar] [CrossRef]
- Drewienkiewicz, A.; Żydek, A.; Trybula, M.; Pstruś, J. Atomic Level Insight into Wetting and Structure of Ag Droplet on Graphene Coated Copper Substrate—Molecular Dynamics versus Experiment. Nanomaterials 2021, 11, 1465. [Google Scholar] [CrossRef]
- Wang, Y.-B.; Wang, X.-D.; Yang, Y.-R.; Chen, M. The Maximum Spreading Factor for Polymer Nanodroplets Impacting a Hydrophobic Solid Surface. J. Phys. Chem. C 2019, 123, 12841–12850. [Google Scholar] [CrossRef]
- Song, F.; Ju, D.; Fan, J.; Chen, Q.; Yang, Q. Deformation hysteresis of a water nano-droplet in an electric field. Eur. Phys. J. E 2019, 42, 120. [Google Scholar] [CrossRef]
- Kwon, T.W.; Park, Y.G.; Park, S.H.; Ha, M.Y. Dynamic Behavior of a Nanosized Water Droplet on the Stepped Surface with a Wetting Gradient. Langmuir 2020, 37, 330–338. [Google Scholar] [CrossRef]
- Pillai, R.; Borg, M.K.; Reese, J.M. Acoustothermal Atomization of Water Nanofilms. Phys. Rev. Lett. 2018, 121, 104502. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Ma, K.; Wang, Q.; Xue, H. The wetting of Pb droplet on the solid Al surface can be promoted by ultrasonic vibration—Molecular dynamics simulation. Mater. Lett. 2020, 264, 127118. [Google Scholar] [CrossRef]
- Pillai, R.; Borg, M.K.; Reese, J.M. Dynamics of Nanodroplets on Vibrating Surfaces. Langmuir 2018, 34, 11898–11904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, J.T.; Wang, B.; Král, P. Nanodroplet Transport on Vibrated Nanotubes. J. Phys. Chem. Lett. 2012, 3, 353–357. [Google Scholar] [CrossRef]
- Sappati, K.K.; Bhadra, S. Piezoelectric Polymer and Paper Substrates: A Review. Sensors 2018, 18, 3605. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Li, P.; Lin, S.-C.S.; Stratton, Z.S.; Nama, N.; Guo, F.; Slotcavage, D.; Mao, X.; Shi, J.; Costanzo, F.; et al. Surface acoustic wave microfluidics. Lab Chip 2013, 13, 3626–3649. [Google Scholar] [CrossRef]
- Li, R.; Li, G.; Hong, W.-C.; Reyes, P.I.; Tang, K.; Yang, K.; Wang, S.-Y.; Ye, H.; Li, Y.; Zhang, L.; et al. Tunable surface acoustic wave device using semiconducting MgZnO and piezoelectric NiZnO dual-layer structure on glass. Smart Mater. Struct. 2018, 27, 085025. [Google Scholar] [CrossRef]
- Johnston, S.R.; Yang, Y.; Cui, Y.-T.; Ma, E.Y.; Kämpfe, T.; Eng, L.M.; Zhou, J.; Chen, Y.-F.; Lu, M.; Shen, Z.-X. Measurement of surface acoustic wave resonances in ferroelectric domains by microwave microscopy. J. Appl. Phys. 2017, 122, 074101. [Google Scholar] [CrossRef]
- Su, R.; Fu, S.; Shen, J.; Chen, Z.; Lu, Z.; Yang, M.; Wang, R.; Zeng, F.; Wang, W.; Song, C.; et al. Enhanced Performance of ZnO/SiO2/Al2O3 Surface Acoustic Wave Devices with Embedded Electrodes. ACS Appl. Mater. Interfaces 2020, 12, 42378–42385. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhou, J.; Zeng, P.; Liu, Y.; Shen, Y.; Yao, W.; Chen, Z.; Wu, J.; Xiong, S.; Chen, Y.; et al. 30 GHz surface acoustic wave transducers with extremely high mass sensitivity. Appl. Phys. Lett. 2020, 116, 123502. [Google Scholar] [CrossRef] [Green Version]
- Fu, S.; Wang, W.; Qian, L.; Li, Q.; Lu, Z.; Shen, J.; Song, C.; Zeng, F.; Pan, F. High-Frequency Surface Acoustic Wave Devices Based on ZnO/SiC Layered Structure. IEEE Electron Device Lett. 2019, 40, 103–106. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, L.; Wang, Q.; Hao, P.; Zheng, X. Wettability behavior of nanodroplets on copper surfaces with hierarchical nanostructures. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 604, 125291. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Fu, T.; Wu, N.; Lu, C.; Wang, J.; Wang, Q. Effect of nanostructure on wettability on copper surface: A molecular dynamic study. Mol. Simul. 2019, 45, 35–39. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J.C. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Valle, C.U.; Kumar, S.; Ramos-Alvarado, B. Investigation on the Wetting Behavior of 3C-SiC Surfaces: Theory and Modeling. J. Phys. Chem. C 2018, 122, 7179–7186. [Google Scholar] [CrossRef]
- Koishi, T.; Yasuoka, K.; Zeng, X.C. Molecular Dynamics Simulation of Water Nanodroplet Bounce Back from Flat and Nanopillared Surface. Langmuir 2017, 33, 10184–10192. [Google Scholar] [CrossRef]
- Ren, H.; Yang, F.; Li, C.; Deng, C. Head-on collision of binary nanodroplets on rough surfaces: Impact velocity dependent spreading dynamics. Appl. Surf. Sci. 2021, 541, 148426. [Google Scholar] [CrossRef]
- Yin, Z.-J.; Ding, Z.-L.; Zhang, W.-F.; Su, R.; Chai, F.-T.; Yu, P. Dynamic behaviors of nanoscale binary water droplets simultaneously impacting on flat surface. Comput. Mater. Sci. 2020, 183, 109814. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, X.; Liu, J.; Liu, Y.; Cai, W.; Chen, J. The study of water wettability on solid surfaces by molecular dynamics simulation. Surf. Sci. 2021, 714, 121916. [Google Scholar] [CrossRef]
- Heinz, H.; Vaia, R.A.; Farmer, B.L.; Naik, R.R. Accurate Simulation of Surfaces and Interfaces of Face-Centered Cubic Metals Using 12−6 and 9−6 Lennard-Jones Potentials. J. Phys. Chem. C 2008, 112, 17281–17290. [Google Scholar] [CrossRef]
- Wu, N.; Zeng, L.; Fu, T.; Wang, Z.; Deng, X. Mechanism of heat transfer enhancement by nanochannels copper plate interface wettability: A molecular dynamics study. Int. J. Therm. Sci. 2021, 159, 106589. [Google Scholar] [CrossRef]
- Bai, P.; Zhou, L.; Du, X. Molecular dynamics simulation of the roles of roughness ratio and surface potential energy in explosive boiling. J. Mol. Liq. 2021, 335, 116169. [Google Scholar] [CrossRef]
- Gao, S.; Liu, W.; Liu, Z. Tuning nanostructured surfaces with hybrid wettability areas to enhance condensation. Nanoscale 2019, 11, 459–466. [Google Scholar] [CrossRef]
- Chen, L.; Wang, S.-Y.; Xiang, X.; Tao, W.-Q. Mechanism of surface nanostructure changing wettability: A molecular dynamics simulation. Comput. Mater. Sci. 2020, 171, 109223. [Google Scholar] [CrossRef]
- Bekele, S.; Evans, O.G.; Tsige, M. Spreading Dynamics of Water Droplets on a Completely Wetting Surface. J. Phys. Chem. C 2020, 124, 20109–20115. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef] [Green Version]
- Song, F.; Ma, L.; Fan, J.; Chen, Q.; Zhang, L.; Li, B.Q. Wetting Behaviors of a Nano-Droplet on a Rough Solid Substrate under Perpendicular Electric Field. Nanomaterials 2018, 8, 340. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhang, K. Dynamic behavior of water droplets impacting on the superhydrophobic surface: Both experimental study and molecular dynamics simulation study. Appl. Surf. Sci. 2019, 498, 143793. [Google Scholar] [CrossRef]
- Cheng, Z.; Du, M.; Lai, H.; Zhang, N.; Sun, K. Super-hydrophobic Copper Surface with Controlled Adhesion Prepared via Ammonia Corrosion. Chem. J. Chin. Univ.-Chin. 2013, 34, 606–609. [Google Scholar] [CrossRef]
- Josserand, C.; Thoroddsen, S.T. Drop Impact on a Solid Surface. Annu. Rev. Fluid Mech. 2016, 48, 365–391. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Zhang, H.; Lin, G. Molecular dynamics simulation on dynamic behaviors of nanodroplets impinging on solid surfaces decorated with nanopillars. Acta Phys. Sin.-Chin. Ed. 2021, 70, 307–316. [Google Scholar] [CrossRef]


















| qO (e) | qH (e) | rOH (Å) | θHOH (°) |
|---|---|---|---|
| −1.0484 | +0.5242 | 0.9572 | 104.52 |
| Atom Type | O | Cu |
|---|---|---|
| ε (kcal/mol) | εO = 0.1628 | εCu = 0.2379 |
| σ (Å) | σO = 3.1644 | σCu = 2.3400 |
| Nanodroplet Radius (Å) | Number of Molecules |
|---|---|
| 35 | 5991 |
| 40 | 8953 |
| 45 | 12,721 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Pan, L.; Xie, X. Molecular Dynamics Simulation on Behaviors of Water Nanodroplets Impinging on Moving Surfaces. Nanomaterials 2022, 12, 247. https://doi.org/10.3390/nano12020247
Zhang H, Pan L, Xie X. Molecular Dynamics Simulation on Behaviors of Water Nanodroplets Impinging on Moving Surfaces. Nanomaterials. 2022; 12(2):247. https://doi.org/10.3390/nano12020247
Chicago/Turabian StyleZhang, Hao, Ling Pan, and Xuqing Xie. 2022. "Molecular Dynamics Simulation on Behaviors of Water Nanodroplets Impinging on Moving Surfaces" Nanomaterials 12, no. 2: 247. https://doi.org/10.3390/nano12020247