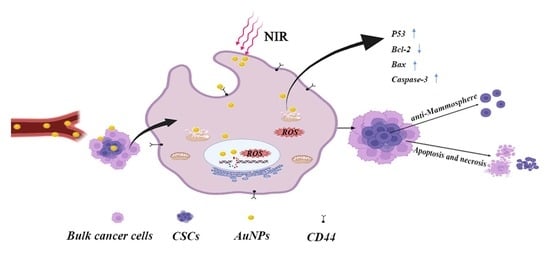
Green Synthesis of Gold Nanoparticles and Study of Their Inhibitory Effect on Bulk Cancer Cells and Cancer Stem Cells in Breast Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells, Materials and Chemicals
2.2. Green Synthesis of HA-Capped AuNPs and Their Characterization
2.3. Level of Cellular Uptake of HA-Capped AuNPs in MDA-MB-231 Cells
2.4. Study of Cytotoxic Effect of HA-Capped AuNPs on MDA-MB-231 and L929 Cells
2.5. Biocompatibility ASSESSMENt by a Hemolysis Test
2.6. Apoptotic Activity of HA-Capped AuNPs and Underlying Mechanism
2.6.1. Apoptosis and Necrosis
2.6.2. Nuclear and Membrane Damage
2.6.3. Mitochondria Morphology and Mitochondrial Membrane Potential (MMP)
2.6.4. ROS Activity
2.6.5. Caspase 3 Activity
2.6.6. Key Markers in Mitochondrial Apoptotic Cascade
2.7. Inhibitory Activity of HA-Capped AuNPs on CSCs: Mammosphere Formation and Viability
2.8. Statistics Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of HA-Capped AuNPs
3.2. Level of Cellular Uptake of HA-Capped AuNPs in MDA-MB-231 Cells
3.3. In Vitro Anti-Cancer Activity, Selectivity and Hemolytic Effect of HA-Capped AuNPs
3.4. Apoptotic Activity of HA-Capped AuNPs
3.5. The Morphology of Nucleus and Integrity of Plasma Membrane
3.6. Mitochondrial Apoptotic Pathway
3.7. Inhibitory Activity of HA-Capped AuNPs on CSC: Mammosphere Formation Capacity and CSC Viability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wu, W.B.; Duan, G.Y. Clinical research progress of double primary cancers of breast and lung with breast cancer as the first primary cancer. Cancer Resprevtreat. 2021, 48, 400–405. [Google Scholar]
- Grady, W.M.; Yu, M.; Markowitz, S.D. Epigenetic alterations in the gastrointestinal tract: Current and emerging use for biomarkers of cancer. Adv. Cancer Res. 2021, 151, 425–468. [Google Scholar]
- Mattiuzzi, C.; Lippi, G. Current cancer epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217–222. [Google Scholar] [CrossRef]
- Lagoa, R.; Silva, J.; Rodrigues, J.R.; Bishayee, A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnol. Adv. 2020, 38, 107382. [Google Scholar] [CrossRef]
- Yan, H.; You, Y.; Li, X.; Liu, L.; Guo, F.; Zhang, Q.; Liu, D.; Tong, Y.; Ding, S.; Wang, J. Preparation of RGD peptide/folate acid double-targeted mesoporous silica nanoparticles and its application in human breast cancer MCF-7 Cells. Front. Pharmacol. 2020, 11, 898. [Google Scholar] [CrossRef]
- Khutale, G.V.; Casey, A. Synthesis and characterization of a multifunctional gold-doxorubicin nanoparticle system for pH triggered intracellular anticancer drug release. Eur. J. Pharm. Biopharm. 2017, 119, 372–380. [Google Scholar] [CrossRef]
- Minko, T.; Dharap, S.S.; Fabbricatore, A.T. Enhancing the efficacy of chemotherapeutic drugs by the suppression of antiapoptotic cellular defense. Cancer Detect. Prev. 2003, 27, 193–202. [Google Scholar] [CrossRef]
- Satapathy, S.R.; Siddharth, S.; Das, D.; Nayak, A.; Kundu, C.N. Enhancement of cytotoxicity and inhibition of angiogenesis in oral cancer stem cells by a hybrid nanoparticle of bioactive quinacrine and silver: Implication of base excision repair cascade. Mol. Pharm. 2015, 12, 4011–4025. [Google Scholar] [CrossRef]
- Meldolesi, J. Cancer stem cells and their vesicles, together with other stem and non-stem cells, govern critical cancer processes: Perspectives for medical development. Int. J. Mol. Sci. 2022, 23, 625. [Google Scholar] [CrossRef]
- Choi, Y.J.; Gurunathan, S.; Kim, J.H. Graphene oxide-silver nanocomposite enhances cytotoxic and apoptotic potential of salinomycin in human ovarian cancer stem cells (OvCSCs): A novel approach for cancer therapy. Int. J. Mol. Sci. 2018, 19, 710. [Google Scholar] [CrossRef]
- Motohara, T.; Yoshida, G.J.; Katabuchi, H. The hallmarks of ovarian cancer stem cells and niches: Exploring their harmonious interplay in therapy resistance. Semin. Cancer Biol. 2021, 77, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.S.; Radharani, N.N.V.; Gorain, M.; Bulbule, A.; Shetti, D.; Roy, G.; Baby, T.; Kundu, G.C. RGD functionalized chitosan nanoparticle mediated targeted delivery of raloxifene selectively suppresses angiogenesis and tumor growth in breast cancer. Nanoscale 2020, 12, 10664–10684. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.; Minz, A.P.; Ashe, S.; Rauta, P.R.; Kumari, M.; Chopra, P.; Nayak, B. Synergistic combination of antioxidants, silver nanoparticles and chitosan in a nanoparticle based formulation: Characterization and cytotoxic effect on MCF-7 breast cancer cell lines. J. Colloid Interface Sci. 2016, 470, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Lomelí-Marroquín, D.; Cruz, D.M.; Nieto-Argüello, A.; Crua, A.V.; Chen, J.; Torres-Castro, A.; Webster, T.J.; Cholula-Díaz, J.L. Starch-mediated synthesis of mono- and bimetallic silver/gold nanoparticles as antimicrobial and anticancer agents. Int. J. Nanomed. 2019, 14, 2171–2190. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Gold nanoparticles for drug delivery and cancer therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Baharara, J.; Namvar, F.; Mousavi, M.; Ramezani, T.; Mohamad, R. Anti-angiogenesis effect of biogenic silver nanoparticles synthesized using saliva officinalis on chick chorioalantoic membrane (CAM). Molecules 2014, 19, 13498–13508. [Google Scholar] [CrossRef]
- Castro-Aceituno, V.; Abbai, R.; Moon, S.S.; Ahn, S.; Mathiyalagan, R.; Kim, Y.-J.; Kim, Y.-J.; Yang, D.C. Pleuropterus multiflorus (Hasuo) mediated straightforward eco-friendly synthesis of silver, gold nanoparticles and evaluation of their anti-cancer activity on A549 lung cancer cell line. Biomed. Pharmacother. 2017, 93, 995–1003. [Google Scholar] [CrossRef]
- Chung, C.Y.-S.; Fung, S.-K.; Tong, K.-C.; Wan, P.-K.; Lok, C.-N.; Huang, Y.; Chen, T.; Che, C.-M. A multi-functional PEGylated gold(iii) compound: Potent anti-cancer properties and self-assembly into nanostructures for drug co-delivery. Chem. Sci. 2017, 8, 1942–1953. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, Z.; Fang, J.; Wang, B.; Lin, Z.; Chen, Z.-S.; Chen, Y.; Zhang, N.; Yang, X.; Gao, W. A multi-functionalized nanocomposite constructed by gold nanorod core with triple-layer coating to combat multidrug resistant colorectal cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110224. [Google Scholar] [CrossRef]
- Ahmad, T.; Sarwar, R.; Iqbal, A.; Bashir, U.; Farooq, U.; Halim, S.A.; Khan, A.; Al-Harrasi, A. Recent advances in combinatorial cancer therapy via multifunctionalized gold nanoparticles. Nanomedicine 2020, 15, 1221–1237. [Google Scholar] [CrossRef]
- Rajendran, I.; Dhandapani, H.; Anantanarayanan, R.; Rajaram, R. Apigenin mediated gold nanoparticle synthesis and their anti-cancer effect on human epidermoid carcinoma (A431) cells. Rsc. Adv. 2015, 5, 51055–51066. [Google Scholar] [CrossRef]
- Seo, J.M.; Kim, E.B.; Hyun, M.S.; Kim, B.B.; Park, T.J. Self-assembly of biogenic gold nanoparticles and their use to enhance drug delivery into cells. Colloid. Surf. B 2015, 135, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Seok, H.Y.; Rejinold, N.S.; Lekshmi, K.M.; Cherukula, K.; Park, I.K.; Kim, Y.C. CD44 targeting biocompatible and biodegradable hyaluronic acid cross-linked zein nanogels for curcumin delivery to cancer cells: In vitro and in vivo evaluation. J. Control. Release 2018, 280, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, J.; Zhu, W.; Sun, C.; Di, D.; Zhang, Y.; Wang, P.; Wang, Z.; Wang, S. Dual-stimuli responsive hyaluronic acid-conjugated mesoporous silica for targeted delivery to CD44-overexpressing cancer cells. Acta Biomater. 2015, 23, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Goltsche, K.; Cheng, L.; Xie, F.; Meng, F.; Deng, C.; Zhong, Z.; Haag, R. Hyaluronic acid-shelled acid-activatable paclitaxel prodrug micelles effectively target and treat CD44-overexpressing human breast tumor xenografts in vivo. Biomaterials 2016, 84, 250–261. [Google Scholar] [CrossRef]
- Noh, I.; Kim, H.-O.; Choi, J.; Choi, Y.; Lee, D.K.; Huh, Y.-M.; Haam, S. Co-delivery of paclitaxel and gemcitabine via CD44-targeting nanocarriers as a prodrug with synergistic antitumor activity against human biliary cancer. Biomaterials 2015, 53, 763–774. [Google Scholar] [CrossRef]
- Luo, M.; Wicha, M.S. Targeting cancer stem cell redox metabolism to enhance therapy responses. Semin. Radiat. Oncol. 2019, 29, 42–54. [Google Scholar] [CrossRef]
- Liang, S.; Li, C.; Zhang, C.; Chen, Y.; Xu, L.; Bao, C.; Wang, X.; Liu, G.; Zhang, F.; Cui, D. CD44v6 monoclonal antibody-conjugated gold nanostars for targeted photoacoustic imaging and plasmonic photothermal therapy of gastric cancer stem-like cells. Theranostics 2015, 5, 970–984. [Google Scholar] [CrossRef]
- Muhammad, N.; Zhao, H.; Song, W.; Gu, M.; Li, Q.; Liu, Y.; Li, C.; Wang, J.; Zhan, H. Silver nanoparticles functionalized paclitaxel nanocrystals enhance overall anti-cancer effect on human cancer cells. Nanotechnology 2021, 32, 085105. [Google Scholar] [CrossRef]
- Wang, J.; Muhammad, N.; Li, T.; Wang, H.; Liu, Y.; Liu, B.; Zhan, H. Hyaluronic acid-coated camptothecin nanocrystals for targeted drug delivery to enhance anticancer efficacy. Mol. Pharm. 2020, 17, 2411–2425. [Google Scholar] [CrossRef]
- Zhan, H.L.; Zhao, H.; Muhammad, N.; Li, T.T.; Liu, Y.J.; Wang, J.H. Lytic peptide-grafted beta-cyclodextrin polymer based nano-scaled drug delivery system with enhanced camptothecin anti-cancer efficacy. Nanotechnology 2020, 31, 075101. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Lin, J.; Cao, J.; Zhang, P.; Bai, Y.; Chen, G.; Chen, K. An exopolysaccharide from Trichoderma pseudokoningii and its apoptotic activity on human leukemia K562 cells. Carbohydr. Polym. 2012, 89, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, E.; Dizaj, S.M.; Rahimpour, E.; Hasanzadeh, A.; Eftekhari, A.; Halajzadeh, J.; Ahmadian, H. Effect of silver nanoparticles in the induction of apoptosis on human hepatocellular carcinoma (HepG2) cell line. Mater. Sci. Eng. C 2018, 93, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, H.; Song, W.; Gu, M.; Liu, Y.; Liu, B.; Zhan, H. Gold nanoparticle-decorated drug nanocrystals for enhancing anticancer efficacy and reversing drug resistance through chemo-/photothermal therapy. Mol. Pharm. 2022, 19, 2518–2534. [Google Scholar] [CrossRef]
- Hwang, S.; Nam, J.; Jung, S.; Song, J.; Doh, H.; Kim, S. Gold nanoparticle-mediated photothermal therapy: Current status and future perspective. Nanomedicine 2014, 9, 2003–2022. [Google Scholar] [CrossRef]
- Da Silva, C.G.; Peters, G.J.; Ossendorp, F.; Cruz, L.J. The potential of multi-compound nanoparticles to bypass drug resistance in cancer. Cancer Chemother. Pharmacol. 2017, 80, 881–894. [Google Scholar] [CrossRef]
- Moses, S.L. Citrate Reduced Gold Nanoparticles Conjugated with Boswellia Sacra and Commiphora Myrrha to Cause Cytotoxicity in Breast Cancer Cells without Harming Health Cells: “We Three Things of Orient Are”. Ph. D. Thesis, Alabama Agricultural and Mechanical University, Normal, AL, USA, 2016. [Google Scholar]
- Cheng, Y.; Meyers, J.D.; Broome, A.M.; Kenney, M.E.; Basilion, J.P.; Burda, C. Deep penetration of a PDT drug into tumors by noncovalent drug-gold nanoparticle conjugates. J. Am. Chem. Soc. 2011, 133, 2583–2591. [Google Scholar] [CrossRef]
- Chugh, H.; Sood, D.; Chandra, I.; Tomar, V.; Dhawan, G.; Chandra, R. Role of gold and silver nanoparticles in cancer nano-medicine. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S1), 1210–1220. [Google Scholar] [CrossRef]
- Tawagi, E.; Massmann, C.; Chibli, H.; Nadeau, L. Differential toxicity of gold-doxorubicin in cancer cells vs. cardiomyocytes as measured by real-time growth assays and fluorescence lifetime imaging microscopy (FLIM). Analyst 2015, 140, 5732–5741. [Google Scholar] [CrossRef] [Green Version]










| HA/Au3+ | Particle Sizes (nm) | PDI | Zeta-Potential (mV) |
|---|---|---|---|
| 1/2 | 32.43 ± 0.23 | 0.057 ± 0.005 | −45.68 ± 0.79 |
| 1/3 | 27.36 ± 0.32 | 0.291 ± 0.030 | −42.05 ± 1.21 |
| 1/4 | 300.20 ± 7.32 | 0.312 ± 0.012 | n.a. |
| Samples | Bulk MDA-MB-231 Cells | CSCs | L929 |
|---|---|---|---|
| CPT | 36.9 ± 1.6 | 223.7 ± 13.3 | 15.9 ± 1.3 |
| HA-capped AuNPs | 34.8 ± 1.8 | 181.1 ± 15.6 | 935.97 ± 30.8 |
| HA-capped AuNPs + L | 22.4 ± 1.4 | 97.7 ± 5.6 | 495.1 ± 25.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Liu, N.; Su, Q.; Lv, Y.; Yang, C.; Zhan, H. Green Synthesis of Gold Nanoparticles and Study of Their Inhibitory Effect on Bulk Cancer Cells and Cancer Stem Cells in Breast Carcinoma. Nanomaterials 2022, 12, 3324. https://doi.org/10.3390/nano12193324
Wang J, Liu N, Su Q, Lv Y, Yang C, Zhan H. Green Synthesis of Gold Nanoparticles and Study of Their Inhibitory Effect on Bulk Cancer Cells and Cancer Stem Cells in Breast Carcinoma. Nanomaterials. 2022; 12(19):3324. https://doi.org/10.3390/nano12193324
Chicago/Turabian StyleWang, Jihui, Na Liu, Qing Su, Yulong Lv, Chang Yang, and Honglei Zhan. 2022. "Green Synthesis of Gold Nanoparticles and Study of Their Inhibitory Effect on Bulk Cancer Cells and Cancer Stem Cells in Breast Carcinoma" Nanomaterials 12, no. 19: 3324. https://doi.org/10.3390/nano12193324