Wavelength Selective Solar Cells Using Triple Cation Perovskite
Abstract
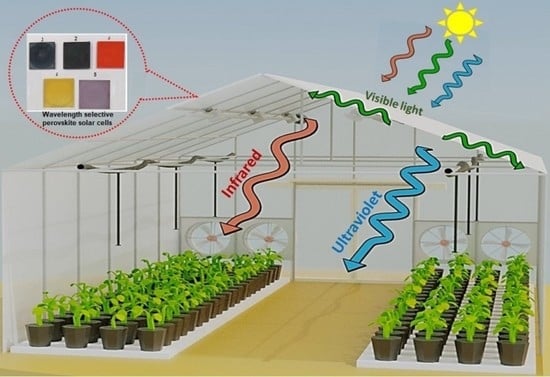
:1. Introduction
2. Experimental Work
2.1. Materials
2.2. Solar Cell Fabrication
2.3. Characterisation
2.4. Modification of Perovskite Composition
3. Results and Discussion
3.1. Optical Properties of Various Compositions of the Perovskite Solar Cell
3.2. Photoluminescence Measurements of Various Compositions of the Perovskite Films
3.3. Structural Properties of Various Compositions of the Perovskite Films
3.4. Current−Voltage Characteristics of Various Compositions of the Perovskite Solar Cell
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stark, G. Light. Encyclopedia Britannica. Available online: https://www.britannica.com/science/light (accessed on 7 May 2021).
- Thompson, E.P.; Bombelli, E.L.; Shubham, S.; Watson, H.; Everard, A.; D’Ardes, V.; Schievano, A.; Bocchi, S.; Zand, N.; Howe, C.J.; et al. Tinted Semi-Transparent Solar Panels Allow Concurrent Production of Crops and Electricity on the Same Cropland. Adv. Energy Mater. 2020, 10, 2001189. [Google Scholar] [CrossRef]
- Hammam, M.; El-Mansy, M.K.; El-Bashir, S.M.; El-Shaarawy, M.G. Performance Evaluation of Thin-Film Solar Concentrators for Greenhouse Applications. Desalination 2007, 209, 244–250. [Google Scholar] [CrossRef]
- Vashishtha, P.; Metin, D.Z.; Cryer, M.E.; Chen, K.; Hodgkiss, J.M.; Gaston, N.; Halpert, J.E. Shape-, Size-, and Composition-Controlled Thallium Lead Halide Perovskite Nanowires and Nanocrystals with Tunable Band Gaps. Chem. Mater. 2018, 30, 2973–2982. [Google Scholar] [CrossRef]
- Eperon, G.E.; Stranks, S.D.; Menelaou, C.; Johnston, M.B.; Herz, L.M.; Snaith, H.J. Formamidinium Lead Trihalide: A Broadly Tunable Perovskite for Efficient Planar Heterojunction Solar Cells. Energy Environ. Sci. 2014, 7, 982–988. [Google Scholar] [CrossRef]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S.I. Chemical Management for Colorful, Efficient, and Stable Inorganic-Organic Hybrid Nanostructured Solar Cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Baikie, T.; Boix, P.P.; Yantara, N.; Mathews, N.; Mhaisalkar, S. Band-Gap Tuning of Lead Halide Perovskites Using a Sequential Deposition Process. J. Mater. Chem. A 2014, 2, 9221–9225. [Google Scholar] [CrossRef]
- Jang, D.M.; Park, K.; Kim, D.H.; Park, J.; Shojaei, F.; Kang, H.S.; Ahn, J.P.; Lee, J.W.; Song, J.K. Reversible Halide Exchange Reaction of Organometal Trihalide Perovskite Colloidal Nanocrystals for Full-Range Band Gap Tuning. Nano Lett. 2015, 15, 5191–5199. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Liu, Z.; Zhang, M.; Li, W.; Zhu, A.; Cai, F.; Zhao, Z.; Zhou, Y. Semitransparent Fully Air Processed Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 17776–17781. [Google Scholar] [CrossRef]
- Kim, G.M.; Tatsuma, T. Semi-Transparent Perovskite Solar Cells Developed by Considering Human Luminosity Function. Sci. Rep. 2017, 7, 4–6. [Google Scholar] [CrossRef]
- Subhani, W.S.; Wang, K.; Du, M.; Wang, X.; Yuan, N.; Ding, J.; Liu, S.F. Anti-Solvent Engineering for Efficient Semitransparent CH3NH3PbBr3 Perovskite Solar Cells for Greenhouse Applications. J. Energy Chem. 2019, 34, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Ramírez Quiroz, C.O.; Bronnbauer, C.; Levchuk, I.; Hou, Y.; Brabec, C.J.; Forberich, K. Coloring Semitransparent Perovskite Solar Cells via Dielectric Mirrors. ACS Nano 2016, 10, 5104–5112. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Pan, X.; Ma, Y.; Li, Y.; Zheng, L.; Zhang, D.; Xu, Q.; Chen, Z.; Wang, S.; Qu, B.; et al. Plasmonic-Enhanced Perovskite Solar Cells Using Alloy Popcorn Nanoparticles. RSC Adv. 2015, 5, 11175–11179. [Google Scholar] [CrossRef]
- Cannavale, A.; Eperon, G.E.; Cossari, P.; Abate, A.; Snaith, H.J.; Gigli, G. Perovskite Photovoltachromic Cells for Building Integration. Energy Environ. Sci. 2015, 8, 1578–1584. [Google Scholar] [CrossRef]
- Gholipour, S.; Saliba, M. Bandgap Tuning and Compositional Exchange for Lead Halide Perovskite Materials; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Brenner, P.; Glöckler, T.; Rueda-Delgado, D.; Abzieher, T.; Jakoby, M.; Richards, B.S.; Paetzold, U.W.; Howard, I.A.; Lemmer, U. Triple Cation Mixed-Halide Perovskites for Tunable Lasers. Opt. Mater. Express 2017, 7, 4082. [Google Scholar] [CrossRef]
- Hayali, A.; Alkaisi, M.M. High Efficiency Perovskite Solar Cells Using DC Sputtered Compact TiO2 electron Transport Layer. EPJ Photovolt. 2021, 12, 2021008. [Google Scholar] [CrossRef]
- Scheidt, R.A.; Kamat, P.V. Temperature-Driven Anion Migration in Gradient Halide Perovskites. J. Chem. Phys. 2019, 151, 134703. [Google Scholar] [CrossRef]
- Albero, J.; Asiri, A.M.; García, H. Influence of the Composition of Hybrid Perovskites on Their Performance in Solar Cells. J. Mater. Chem. A 2016, 4, 4353–4364. [Google Scholar] [CrossRef]
- Atourki, L.; Vega, E.; Mollar, M.; Marí, B.; Kirou, H.; Bouabid, K.; Ihlal, A. Impact of Iodide Substitution on the Physical Properties and Stability of Cesium Lead Halide Perovskite Thin Films CsPbBr3−xIx(0 ≤ x ≤ 1). J. Alloys Compd. 2017, 702, 404–409. [Google Scholar] [CrossRef]
- Ghobadi, N. Band Gap Determination Using Absorption Spectrum Fitting Procedure. Int. Nano Lett. 2013, 3, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Saliba, M.; Matsui, T.; Seo, J.Y.; Domanski, K.; Correa-Baena, J.P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-Containing Triple Cation Perovskite Solar Cells: Improved Stability, Reproducibility and High Efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, S.; Jin, S.; Li, H.; Zhai, T. Crystal Organometal Halide Perovskites with Promising Optoelectronic Applications. J. Mater. Chem. C 2015, 4, 11–27. [Google Scholar] [CrossRef]
- Song, D.; Cui, P.; Wang, T.; Wei, D.; Li, M.; Cao, F.; Yue, X.; Fu, P.; Li, Y.; He, Y.; et al. Managing Carrier Lifetime and Doping Property of Lead Halide Perovskite by Postannealing Processes for Highly Efficient Perovskite Solar Cells. J. Phys. Chem. C 2015, 119, 22812–22819. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Xu, F.; Wang, Y.; Li, G.; Yang, Y.; Zhao, Y. CH3NH3Cl Assisted Solvent Engineering for Highly Crystallized and Large Grain Size Mixed-Composition (FAPbI3)0.85(MAPbBr3)0.15 Perovskites. Crystals 2017, 7, 272. [Google Scholar] [CrossRef]
- Li, Z.; Yang, M.; Park, J.S.; Wei, S.H.; Berry, J.J.; Zhu, K. Stabilizing Perovskite Structures by Tuning Tolerance Factor: Formation of Formamidinium and Cesium Lead Iodide Solid-State Alloys. Chem. Mater. 2016, 28, 284–292. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, W.; Huang, Y.; Wen, F.; Yu, H.; Li, Y.; Wang, Q.; Peng, C.; Ma, Z.; Hu, T.; et al. Effects of Annealing Time on Triple Cation Perovskite Films and Their Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 29344–29356. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Okada, H.; Oku, T. Role of Bromine Doping on the Photovoltaic Properties and Microstructures of CH3NH3PbI3 Perovskite Solar Cells. AIP Conf. Proc. 2016, 1709, 4941221. [Google Scholar] [CrossRef] [Green Version]













| Sample Composition NO. | Description | FAI (M) | FABr (M) | FACl (M) | MAI (M) | MABr (M) | MACl (M) | PbI2 (M) | PbBr2 (M) | PbCl2 (M) | CsI (%) | CsBr (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | (CsI)0.05[FAMAPb(I0.85Br0.15)3]0.95 | 1 | - | - | - | 0.2 | - | 1.1 | 0.2 | - | 5 | - |
| 2 | (CsI)0.05[FAMAPb(I)3]0.95 | 1 | - | - | 0.2 | - | - | 1.3 | - | - | 5 | - |
| 3 | (CsBr)0.05[FAMAPb(Br0.85I0.15)3]0.95 | - | 1 | - | 0.2 | - | - | 0.2 | 1.1 | - | - | 5 |
| 4 | (CsBr)0.05[FAMAPb(Br0.85Cl0.15)3]0.95 | - | 1 | - | - | - | 0.2 | - | 1.1 | 0.2 | - | 5 |
| 5 | (CsI)0.05[FAMAPb(I0.5Br0.5)3]0.95 | 1 | - | - | - | 0.2 | - | 0.63 | 0.79 | - | 5 | - |
| Sample Composition NO. | Description | Efficiency (%) | Grain Size (nm) | Energy Gap (eV) | Lifetime (ns) |
|---|---|---|---|---|---|
| 1 | (CsI)0.05[FAMAPb(I0.85Br0.15)3]0.95 | 15.4 | 190 | 1.63 | 173.99 |
| 2 | (CsI)0.05[FAMAPb(I)3]0.95 | 13.3 | 221 | 1.52 | 158.94 |
| 3 | (CsBr)0.05[FAMAPb(Br0.85I0.15)3]0.95 | 4.2 | 193 | 2.12 | 96.28 |
| 4 | (CsBr)0.05[FAMAPb(Br0.85Cl0.15)3]0.95 | 3.5 | 257 | 2.3 | 103.4 |
| 5 | (CsI)0.05[FAMAPb(I0.5Br0.5)3]0.95 | 9.1 | 249 | 1.98 | 142.4 |
| Sample Description | Sweep Direction | PCE (%) | H.I (%) | FF (%) | Voc (mV) | Jsc (mA/cm2) | Vmax (mV) | Jmax (mA/cm2) | Isc (mA) | Rshunt (Ω∙cm2) | Rseries (Ω∙cm2) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Forward | 15 | 8.5 | 53 | 1049 | 26.4 | 724 | 20.3 | 9.4 | 1305 | 9.3 |
| Backward | 16.4 | 60 | 1052 | 25.4 | 780 | 20.4 | 9.07 | 2024 | 8.6 | ||
| 2 | Forward | 12.5 | 10.7 | 53 | 1005 | 23.3 | 696 | 17.9 | 8.4 | 975 | 11.2 |
| Backward | 14 | 53.9 | 1003 | 25.9 | 696 | 20 | 9.3 | 1725 | 9.7 | ||
| 3 | Forward | 4.2 | 5 | 56 | 939 | 7.8 | 696 | 5.94 | 2.8 | 532 | 27.3 |
| Backward | 4 | 56 | 928 | 7.7 | 668 | 6.02 | 2.7 | 2681 | 23.2 | ||
| 4 | Forward | 2.5 | 28 | 44.8 | 973 | 5.81 | 691 | 3.64 | 2.1 | 417 | 39.6 |
| Backward | 3.5 | 55.3 | 1013 | 6.3 | 754 | 4.69 | 2.2 | 16776 | 30.7 | ||
| 5 | Forward | 8.3 | 16 | 50 | 970 | 17.22 | 612 | 13.58 | 6.14 | 508 | 20 |
| Backward | 9.9 | 58 | 1046 | 16.2 | 780 | 12.6 | 5.8 | 976 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayali, A.; Reeves, R.J.; Alkaisi, M.M. Wavelength Selective Solar Cells Using Triple Cation Perovskite. Nanomaterials 2022, 12, 3299. https://doi.org/10.3390/nano12193299
Hayali A, Reeves RJ, Alkaisi MM. Wavelength Selective Solar Cells Using Triple Cation Perovskite. Nanomaterials. 2022; 12(19):3299. https://doi.org/10.3390/nano12193299
Chicago/Turabian StyleHayali, Ahmed, Roger J. Reeves, and Maan M. Alkaisi. 2022. "Wavelength Selective Solar Cells Using Triple Cation Perovskite" Nanomaterials 12, no. 19: 3299. https://doi.org/10.3390/nano12193299