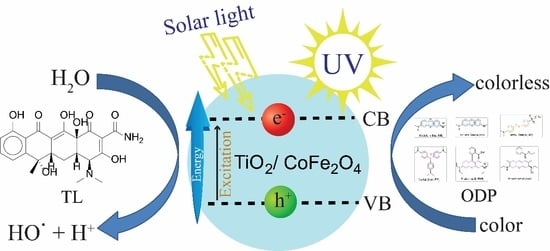
Magnetic TiO2/CoFe2O4 Photocatalysts for Degradation of Organic Dyes and Pharmaceuticals without Oxidants
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Material Preparation
2.2.1. Synthesis of TiO2 Nanoparticles
2.2.2. Synthesis of CoFe2O4
2.2.3. Synthesis of CoFe2O4/TiO2 Composites
2.3. Characterization of Samples
3. Results and Discussion
3.1. Structural Analysis
3.2. Morphological Analysis
3.3. Surface Analysis
3.4. Optical Analysis
3.5. Magnetization Analysis
3.6. Photocatalytic Oxidation Activity
3.6.1. Photocatalytic Degradation of ODP under UV Light
3.6.2. Photocatalytic Degradation of TL under UV and Visible Light
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ali, I.; Kim, J.-O. Visible-light-assisted photocatalytic activity of bismuth-TiO2 nanotube composites for chromium reduction and dye degradation. Chemosphere 2018, 207, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Mittal, A.; Mittal, J.; Malviya, A.; Kaur, D.; Gupta, V. Adsorption of hazardous dye crystal violet from wastewater by waste materials. J. Colloid Interface Sci. 2009, 343, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.K.; Amin, K.; Ahmed, J.; Elias, M.; Mahiuddin, M. Removal of toxic methyl orange by a cost-free and eco-friendly adsorbent: Mechanism, phytotoxicity, thermodynamics, and kinetics. S. Afr. J. Chem. Eng. 2022, 40, 195–208. [Google Scholar] [CrossRef]
- Chandrika, K.; Chaudhary, A.; Mareedu, T.; Sirisha, U.; Vangalapati, M. Adsorptive removal of acridine orange dye by green tea/copper-activated carbon nanoparticles (Gt/Cu-AC np). Mater. Today Proc. 2021, 44, 2283–2289. [Google Scholar] [CrossRef]
- Ahmad, M.; Rehman, W.; Khan, M.M.; Qureshi, M.T.; Gul, A.; Haq, S.; Ullah, R.; Rab, A.; Menaa, F. Phytogenic fabrication of ZnO and gold decorated ZnO nanoparticles for photocatalytic degradation of Rhodamine B. J. Environ. Chem. Eng. 2021, 9, 104725. [Google Scholar] [CrossRef]
- Pal, U.; Sandoval, A.; Madrid, S.I.U.; Corro, G.; Sharma, V.; Mohanty, P. Mixed titanium, silicon, and aluminum oxide nanostructures as novel adsorbent for removal of rhodamine 6G and methylene blue as cationic dyes from aqueous solution. Chemosphere 2016, 163, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, S.; Ahmed, S.; Lo, I.M.; Singh, S.; Sridharan, K. Rapid sonochemical synthesis of copper doped ZnO grafted on graphene as a multi-component hierarchically structured visible-light-driven photocatalyst. Mater. Res. Bull. 2021, 140, 111290. [Google Scholar] [CrossRef]
- Huyen, N.T.K.; Pham, T.-D.; Cam, N.T.D.; Van Quan, P.; Van Noi, N.; Hanh, N.T.; Tung, M.H.T.; Dao, V.-D. Fabrication of titanium doped BiVO4 as a novel visible light driven photocatalyst for degradation of residual tetracycline pollutant. Ceram. Int. 2021, 47, 34253–34259. [Google Scholar] [CrossRef]
- Ibrahim, I.; Belessiotis, G.V.; Arfanis, M.K.; Athanasekou, C.; Philippopoulos, A.I.; Mitsopoulou, C.A.; Romanos, G.E.; Falaras, P. Surfactant Effects on the Synthesis of Redox Bifunctional V2O5 Photocatalysts. Materials 2020, 13, 4665. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Ibrahim, I.; Salama, T.M. Rational design of manganese ferrite-graphene hybrid photocatalysts: Efficient water splitting and effective elimination of organic pollutants. Appl. Catal. A Gen. 2016, 524, 182–191. [Google Scholar] [CrossRef]
- Belessiotis, G.V.; Kontos, A.G. Plasmonic silver (Ag)-based photocatalysts for H2 production and CO2 conversion: Review, analysis and perspectives. Renew. Energy 2022, 195, 497–515. [Google Scholar] [CrossRef]
- Liao, G.; Li, C.; Liu, S.-Y.; Fang, B.; Yang, H. Emerging frontiers of Z-scheme photocatalytic systems. Trends Chem. 2021, 4, 111–127. [Google Scholar] [CrossRef]
- Ibrahim, I.; Athanasekou, C.; Manolis, G.; Kaltzoglou, A.; Nasikas, N.K.; Katsaros, F.; Devlin, E.; Kontos, A.G.; Falaras, P. Photocatalysis as an advanced reduction process (ARP): The reduction of 4-nitrophenol using titania nanotubes-ferrite nanocomposites. J. Hazard. Mater. 2018, 372, 37–44. [Google Scholar] [CrossRef]
- Ibrahim, I.; Belessiotis, G.V.; Antoniadou, M.; Kaltzoglou, A.; Sakellis, E.; Katsaros, F.; Sygellou, L.; Arfanis, M.K.; Salama, T.M.; Falaras, P. Silver decorated TiO2/g-C3N4 bifunctional nanocomposites for photocatalytic elimination of water pollutants under UV and artificial solar light. Results Eng. 2022, 14, 100470. [Google Scholar] [CrossRef]
- Sridharan, K.; Shenoy, S.; Kumar, S.; Terashima, C.; Fujishima, A.; Pitchaimuthu, S. Advanced Two-Dimensional Heterojunction Photocatalysts of Stoichiometric and Non-Stoichiometric Bismuth Oxyhalides with Graphitic Carbon Nitride for Sustainable Energy and Environmental Applications. Catalysts 2021, 11, 426. [Google Scholar] [CrossRef]
- Abdel-Khalek, E.; Ibrahim, I.; Salama, T.M.; Elseman, A.M.; Mohamed, M.M. Structural, optical, dielectric and magnetic properties of Bi1−xLaxFeO3 nanoparticles. J. Magn. Magn. Mater. 2018, 465, 309–315. [Google Scholar] [CrossRef]
- Abdel-Khalek, E.K.; Ibrahim, I.; Salama, T.M. Dielectric anomaly in the microwave region and exchange bias effect in LaFeO3 nanoparticles at room temperature. Ferroelectrics 2019, 550, 210–219. [Google Scholar] [CrossRef]
- Abdel-Khalek, E.K.; Ibrahim, I.; Salama, T.M.; Elseman, A. Study of the optical, dielectric and magnetic properties of the Bi0.75La0.25FeO3 sample. Ferroelectrics 2020, 558, 150–164. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.-G.; Wang, X.-X.; Nie, G.-D.; Zhang, J.; Zhang, S.-X.; Cao, N.; Yan, S.-Y.; Long, Y.-Z. Highly flexible Fe2O3/TiO2 composite nanofibers for photocatalysis and utraviolet detection. J. Phys. Chem. Solids 2018, 121, 236–246. [Google Scholar] [CrossRef]
- Sohail, M.; Xue, H.; Jiao, Q.; Li, H.; Khan, K.; Wang, S.; Feng, C.; Zhao, Y. Synthesis of well-dispersed TiO2/CNTs@CoFe2O4 nanocomposites and their photocatalytic properties. Mater. Res. Bull. 2018, 101, 83–89. [Google Scholar] [CrossRef]
- Wu, P.-F.; Xue, Q.; Wang, T.-Y.; Li, S.-J.; Li, G.-P.; Xue, G.-L. A PW12/Ag functionalized mesoporous silica-coated magnetic Fe3O4 core–shell composite as an efficient and recyclable photocatalyst. Dalton Trans. 2020, 50, 578–586. [Google Scholar] [CrossRef]
- Belessiotis, G.V.; Falara, P.P.; Ibrahim, I.; Kontos, A.G. Magnetic Metal Oxide-Based Photocatalysts with Integrated Silver for Water Treatment. Materials 2022, 15, 4629. [Google Scholar] [CrossRef]
- Ibrahim, I.; Ali, I.O.; Salama, T.M.; Bahgat, A.; Mohamed, M.M. Synthesis of magnetically recyclable spinel ferrite (MFe2O4, M = Zn, Co, Mn) nanocrystals engineered by sol gel-hydrothermal technology: High catalytic performances for nitroarenes reduction. Appl. Catal. B Environ. 2016, 181, 389–402. [Google Scholar] [CrossRef]
- Belessiotis, G.V.; Papadokostaki, K.G.; Favvas, E.P.; Efthimiadou, E.K.; Karellas, S. Preparation and investigation of distinct and shape stable paraffin/SiO2 composite PCM nanospheres. Energy Convers. Manag. 2018, 168, 382–394. [Google Scholar] [CrossRef]
- Ye, T.; Chen, W.; Xu, H.; Geng, N.; Cai, Y. Preparation of TiO2/graphene composite with appropriate N-doping ratio for humic acid removal. J. Mater. Sci. 2017, 53, 613–625. [Google Scholar] [CrossRef]
- Abdelhaleem, A.; Chu, W. Photodegradation of 4-chlorophenoxyacetic acid under visible LED activated N-doped TiO2 and the mechanism of stepwise rate increment of the reused catalyst. J. Hazard. Mater. 2017, 338, 491–501. [Google Scholar] [CrossRef]
- Attia, Y.A.; Altalhi, T.A. Low-cost synthesis of titanium dioxide anatase nanoclusters as advanced materials for hydrogen photoproduction. Res. Chem. Intermed. 2017, 43, 4051–4062. [Google Scholar] [CrossRef]
- Belessiotis, G.; Arfanis, M.; Kaltzoglou, A.; Likodimos, V.; Raptis, Y.; Falaras, P.; Kontos, A. Temperature effects on the vibrational properties of the Cs2SnX6 ‘defect’ perovskites (X = I, Br, Cl). Mater. Chem. Phys. 2021, 267, 124679. [Google Scholar] [CrossRef]
- Belessiotis, G.; Arfanis, M.; Kaltzoglou, A.; Likodimos, V.; Raptis, Y.; Falaras, P.; Kontos, A. Temperature dependence of the vibrational and emission spectra in the 0D vacancy-ordered Cs2SnI6 perovskite. Mater. Today Proc. 2022. [Google Scholar] [CrossRef]
- Stathopoulos, N.; Belessiotis, G.; Oikonomou, P.; Papanicolaou, E. Experimental investigation of thermal degradation of phase change materials for medium-temperature thermal energy storage and tightness during cycling inside metal spheres. J. Energy Storage 2020, 31, 101618. [Google Scholar] [CrossRef]
- Arabatzis, I.; Antonaraki, S.; Stergiopoulos, T.; Hiskia, A.; Papaconstantinou, E.; Bernard, M.; Falaras, P. Preparation, characterization and photocatalytic activity of nanocrystalline thin film TiO2 catalysts towards 3,5-dichlorophenol degradation. J. Photochem. Photobiol. A Chem. 2002, 149, 237–245. [Google Scholar] [CrossRef]
- Bhowmik, R.; Kazhugasalamoorthy, S.; Sinha, A. Role of initial heat treatment of the ferrite component on magnetic properties in the composite of ferrimagnetic Co1.75Fe1.25O4 ferrite and non-magnetic BaTiO3 oxide. J. Magn. Magn. Mater. 2017, 444, 451–466. [Google Scholar] [CrossRef]
- Ali, T.; Ahmed, A.; Alam, U.; Uddin, I.; Tripathi, P.; Muneer, M. Enhanced photocatalytic and antibacterial activities of Ag-doped TiO2 nanoparticles under visible light. Mater. Chem. Phys. 2018, 212, 325–335. [Google Scholar] [CrossRef]
- Abroushan, E.; Farhadi, S.; Zabardasti, A. Ag3PO4/CoFe2O4 magnetic nanocomposite: Synthesis, characterization and applications in catalytic reduction of nitrophenols and sunlight-assisted photocatalytic degradation of organic dye pollutants. RSC Adv. 2017, 7, 18293–18304. [Google Scholar] [CrossRef]
- Hernández-Gordillo, A.; Arroyo, M.; Zanella, R.; Rodríguez-González, V. Photoconversion of 4-nitrophenol in the presence of hydrazine with AgNPs-TiO2 nanoparticles prepared by the sol–gel method. J. Hazard. Mater. 2014, 268, 84–91. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Q.; Li, X.; Wang, D. Enhanced photocatalytic activity of degrading short chain chlorinated paraffins over reduced graphene oxide/CoFe2O4/Ag nanocomposite. J. Colloid Interface Sci. 2016, 479, 89–97. [Google Scholar] [CrossRef]
- Gao, Y.; Wong, K.Y.; Ahiabu, A.; Serpe, M.J. Sequential and controlled release of small molecules from poly(N-isopropylacrylamide) microgel-based reservoir devices. J. Mater. Chem. B 2016, 4, 5144–5150. [Google Scholar] [CrossRef]
- Shen, X. 10—Molecularly Imprinted Photocatalysts. In Molecularly Imprinted Catalysts; Elsevier: Amsterdam, The Netherlands, 2016; pp. 211–228. [Google Scholar] [CrossRef]
- Boumediene, M.; Benaïssa, H.; George, B.; Molina, S.; Merlin, A. Effects of pH and ionic strength on methylene blue removal from synthetic aqueous solutions by sorption onto orange peel and desorption study. J. Mater. Environ. Sci. 2018, 9, 1700–1711. [Google Scholar] [CrossRef]
- Chiang, L.-F.; Doong, R.-A. Enhanced photocatalytic degradation of sulfamethoxazole by visible-light-sensitive TiO2 with low Cu addition. Sep. Purif. Technol. 2015, 156, 1003–1010. [Google Scholar] [CrossRef]
- Ghosh, B.K.; Moitra, D.; Chandel, M.; Ghosh, N.N. Preparation of TiO2/Cobalt Ferrite/Reduced Graphene Oxide Nanocomposite Based Magnetically Separable Catalyst with Improved Photocatalytic Activity. J. Nanosci. Nanotechnol. 2017, 17, 4694–4703. [Google Scholar] [CrossRef]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, I.; Belessiotis, G.V.; Elseman, A.M.; Mohamed, M.M.; Ren, Y.; Salama, T.M.; Mohamed, M.B.I. Magnetic TiO2/CoFe2O4 Photocatalysts for Degradation of Organic Dyes and Pharmaceuticals without Oxidants. Nanomaterials 2022, 12, 3290. https://doi.org/10.3390/nano12193290
Ibrahim I, Belessiotis GV, Elseman AM, Mohamed MM, Ren Y, Salama TM, Mohamed MBI. Magnetic TiO2/CoFe2O4 Photocatalysts for Degradation of Organic Dyes and Pharmaceuticals without Oxidants. Nanomaterials. 2022; 12(19):3290. https://doi.org/10.3390/nano12193290
Chicago/Turabian StyleIbrahim, Islam, George V. Belessiotis, Ahmed Mourtada Elseman, Mohamed Mokhtar Mohamed, Yatao Ren, Tarek M. Salama, and Mahmoud Basseem I. Mohamed. 2022. "Magnetic TiO2/CoFe2O4 Photocatalysts for Degradation of Organic Dyes and Pharmaceuticals without Oxidants" Nanomaterials 12, no. 19: 3290. https://doi.org/10.3390/nano12193290