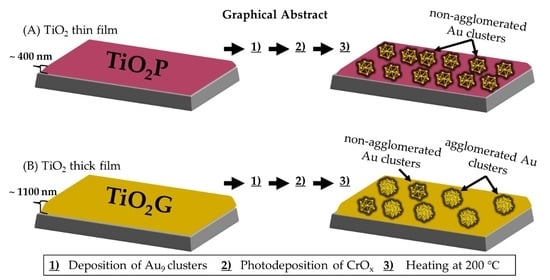
Effect of TiO2 Film Thickness on the Stability of Au9 Clusters with a CrOx Layer
Abstract
:1. Introduction
2. Experimental Methods and Techniques
2.1. Material and Sample Preparation
2.1.1. Preparation of TiO2 Films
2.1.2. Deposition of Au9 Clusters
2.1.3. Photodeposition of CrOx Layer
2.1.4. Heat Treatment
2.2. Characterization Methods
2.2.1. Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy (SEM-EDAX)
2.2.2. X-ray Diffraction (XRD)
2.2.3. Laser Scanning Confocal Microscope (LSCM)
2.2.4. X-ray Photoelectron Spectroscopy (XPS)
3. Results and Discussion
3.1. Influence of the Thickness of the TiO2 Films
3.2. Determination of the TiO2 Film Thickness
3.3. Crystal Structure of the TiO2P and TiO2G before and after Heating
3.4. Morphology of the TiO2P and TiO2G Layer before and after Heating
3.5. Au9 Clusters on TiO2P and TiO2G; a Probe for Mobility during Heating
3.6. XPS of TiO2P Sample
3.7. XPS of TiO2G Sample
3.8. Effect of the TiO2 Film Thickness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 For Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Brinker, C.J.; Harrington, M.S. Sol-gel Derived Antireflective Coatings for Silicon. Sol. Energy Mater. 1981, 5, 159–172. [Google Scholar] [CrossRef]
- Lottiaux, M.; Boulesteix, C.; Nihoul, G.; Varnier, F.; Flory, F.; Galindo, R.; Pelletier, E. Morphology and Structure of TiO2 Thin Layers vs. Thickness and Substrate Temperature. Thin Solid Film. 1989, 170, 107–126. [Google Scholar] [CrossRef]
- Yeung, K.S.; Lam, Y.W. A Simple Chemical Vapour Deposition Method for Depositing Thin TiO2 Films. Thin Solid Film. 1983, 109, 169–178. [Google Scholar] [CrossRef]
- Aarik, J.; Aidla, A.; Uustare, T.; Kukli, K.; Sammelselg, V.; Ritala, M.; Leskelä, M. Atomic Layer Deposition of TiO2 Thin Films from TiI4 and H2O. Appl. Surf. Sci. 2002, 193, 277–286. [Google Scholar] [CrossRef]
- Dakka, A.; Lafait, J.; Abd-Lefdil, M.; Sella, C. Optical Study of Titanium Dioxide Thin Films Prepared by RF Sputtering. Moroc. J. Condens. Matter 1999, 2, 153–156. [Google Scholar]
- Tanemura, S.; Miao, L.; Wunderlich, W.; Tanemura, M.; Mori, Y.; Toh, S.; Kaneko, K. Fabrication and Characterization of Anatase/Rutile–TiO2 Thin Films by Magnetron Sputtering: A Review. Sci. Technol. Adv. Mater. 2005, 6, 11–17. [Google Scholar] [CrossRef]
- Miao, L.; Jin, P.; Kaneko, K.; Terai, A.; Nabatova-Gabain, N.; Tanemura, S. Preparation and characterization of polycrystalline anatase and rutile TiO2 thin films by rf magnetron sputtering. Appl. Surf. Sci. 2003, 212–213, 255–263. [Google Scholar] [CrossRef]
- Wang, T.M.; Zheng, S.K.; Hao, W.C.; Wang, C. Studies on photocatalytic activity and transmittance spectra of TiO2 thin films prepared by r.f. magnetron sputtering method. Surf. Coat. Technol. 2002, 155, 141–145. [Google Scholar] [CrossRef]
- Ye, Q.; Liu, P.Y.; Tang, Z.F.; Zhai, L. Hydrophilic properties of nano-TiO2 thin films deposited by RF magnetron sputtering. Vacuum 2007, 81, 627–631. [Google Scholar] [CrossRef]
- Huang, C.H.; Tsao, C.C.; Hsu, C.Y. Study on the photocatalytic activities of TiO2 films prepared by reactive RF sputtering. Ceram. Int. 2011, 37, 2781–2788. [Google Scholar] [CrossRef]
- Yoo, D.; Kim, I.; Kim, S.; Hahn, C.H.; Lee, C.; Cho, S. Effects of annealing temperature and method on structural and optical properties of TiO2 films prepared by RF magnetron sputtering at room temperature. Appl. Surf. Sci. 2007, 253, 3888–3892. [Google Scholar] [CrossRef]
- Hou, Y.-Q.; Zhuang, D.-M.; Zhang, G.; Zhao, M.; Wu, M.-S. Influence of annealing temperature on the properties of titanium oxide thin film. Appl. Surf. Sci. 2003, 218, 98–106. [Google Scholar] [CrossRef]
- Grilli, M.L.; Yilmaz, M.; Aydogan, S.; Cirak, B.B. Room temperature deposition of XRD-amorphous TiO2 thin films: Investigation of device performance as a function of temperature. Ceram. Int. 2018, 44, 11582–11590. [Google Scholar] [CrossRef]
- Mathews, N.R.; Morales, E.R.; Cortés-Jacome, M.A.; Toledo Antonio, J.A. TiO2 Thin Films—Influence of Annealing Temperature on Structural, Optical and Photocatalytic Properties. Sol. Energy 2009, 83, 1499–1508. [Google Scholar] [CrossRef]
- Meng, F.; Xiao, L.; Sun, Z. Thermo-induced hydrophilicity of nano-TiO2 thin films prepared by RF magnetron sputtering. J. Alloy. Compd. 2009, 485, 848–852. [Google Scholar] [CrossRef]
- Taherniya, A.; Raoufi, D. Thickness dependence of structural, optical and morphological properties of sol-gel derived TiO2 thin film. Mater. Res. Express 2018, 6, 016417. [Google Scholar] [CrossRef]
- Çörekçi, Ş.; Kizilkaya, K.; Asar, T.; Öztürk, M.; Çakmak, M.; Ozcelik, S. Effects of Thermal Annealing and Film Thickness on the Structural and Morphological Properties of Titanium Dioxide Films. Acta Phys. Pol. A 2012, 121, 247–248. [Google Scholar] [CrossRef]
- Pichugina, D.A.; Kuz’menko, N.E.; Shestakov, A.F. Ligand-protected gold clusters: The structure, synthesis and applications. Russ. Chem. Rev. 2015, 84, 1114–1144. [Google Scholar] [CrossRef]
- Adnan, R.H.; Madridejos, J.M.L.; Alotabi, A.S.; Metha, G.F.; Andersson, G.G. A Review of State of the Art in Phosphine Ligated Gold Clusters and Application in Catalysis. Adv. Sci. 2022, 2022, 2105692. [Google Scholar] [CrossRef] [PubMed]
- Daughtry, J.; Alotabi, A.S.; Howard-Fabretto, L.; Andersson, G.G. Composition and Properties of RF-Sputter Deposited Titanium Dioxide Thin Films. Nanoscale Adv. 2021, 3, 1077–1086. [Google Scholar] [CrossRef]
- Yuan, X.; Ye, Y.; Lian, M.; Wei, Q. Structural Coloration of Polyester Fabrics Coated with Al/TiO2 Composite Films and Their Anti-Ultraviolet Properties. Materials 2018, 11, 1011. [Google Scholar] [CrossRef] [PubMed]
- Diamanti, M.V.; Del Curto, B.; Pedeferri, M. Interference Colors of Thin Oxide Layers on Titanium. Color Res. Appl. 2008, 33, 221–228. [Google Scholar] [CrossRef]
- Wen, F.; Englert, U.; Gutrath, B.; Simon, U. Crystal Structure, Electrochemical and Optical Properties of [Au9(PPh3)8](NO3)3. Eur. J. Inorg. Chem. 2008, 2008, 106–111. [Google Scholar] [CrossRef]
- Alotabi, A.S.; Gibson, C.T.; Metha, G.F.; Andersson, G.G. Investigation of the Diffusion of Cr2O3 into Different Phases of TiO2 upon Annealing. ACS Appl. Energy Mater. 2021, 4, 322–330. [Google Scholar] [CrossRef]
- Acres, R.G.; Ellis, A.V.; Alvino, J.; Lenahan, C.E.; Khodakov, D.A.; Metha, G.F.; Andersson, G.G. Molecular Structure of 3-Aminopropyltriethoxysilane Layers Formed on Silanol-Terminated Silicon Surfaces. J. Phys. Chem. C 2012, 116, 6289–6297. [Google Scholar] [CrossRef]
- Alotabi, A.S.; Yin, Y.; Redaa, A.; Tesana, S.; Metha, G.F.; Andersson, G.G. Cr2O3 layer inhibits agglomeration of phosphine-protected Au9 clusters on TiO2 films. J. Chem. Phys. 2021, 155, 164702. [Google Scholar] [CrossRef]
- Ali, S.; Granbohm, H.; Lahtinen, J.; Hannula, S.-P. Titania Nanotubes Prepared by Rapid Breakdown Anodization for Photocatalytic Decolorization of Organic Dyes under UV and Natural Solar Light. Nanoscale Res. Lett. 2018, 13, 179. [Google Scholar] [CrossRef]
- Chandra Sekhar, M.; Kondaiah, P.; Jagadeesh Chandra, S.V.; Mohan Rao, G.; Uthanna, S. Substrate temperature influenced physical properties of silicon MOS devices with TiO2 gate dielectric. Surf. Interface Anal. 2012, 44, 1299–1304. [Google Scholar] [CrossRef]
- Anderson, D.P.; Alvino, J.F.; Gentleman, A.; Al Qahtani, H.; Thomsen, L.; Polson, M.I.; Metha, G.F.; Golovko, V.B.; Andersson, G.G. Chemically-Synthesised, Atomically-Precise Gold Clusters Deposited and Activated on Titania. Phys. Chem. Chem. Phys. 2013, 15, 3917–3929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, D.P.; Adnan, R.H.; Alvino, J.F.; Shipper, O.; Donoeva, B.; Ruzicka, J.-Y.; Al Qahtani, H.; Harris, H.H.; Cowie, B.; Aitken, J.B. Chemically Synthesised Atomically Precise Gold Clusters Deposited and Activated on Titania. Part II. Phys. Chem. Chem. Phys. 2013, 15, 14806–14813. [Google Scholar] [CrossRef] [PubMed]
- Al Qahtani, H.S.; Kimoto, K.; Bennett, T.; Alvino, J.F.; Andersson, G.G.; Metha, G.F.; Golovko, V.B.; Sasaki, T.; Nakayama, T. Atomically resolved structure of ligand-protected Au9 clusters on TiO2 nanosheets using aberration-corrected STEM. J. Chem. Phys. 2016, 144, 114703. [Google Scholar] [CrossRef] [PubMed]
- Al Qahtani, H.S.; Metha, G.F.; Walsh, R.B.; Golovko, V.B.; Andersson, G.G.; Nakayama, T. Aggregation Behavior of Ligand-Protected Au9 Clusters on Sputtered Atomic Layer Deposition TiO2. J. Phys. Chem. C 2017, 121, 10781–10789. [Google Scholar] [CrossRef]
- Howard-Fabretto, L.; Andersson, G.G. Metal Clusters on Semiconductor Surfaces and Application in Catalysis with a Focus on Au and Ru. Adv. Mater. 2020, 32, 1904122. [Google Scholar] [CrossRef] [PubMed]
- Alotabi, A.S.; Osborn, D.J.; Ozaki, S.; Kataoka, Y.; Negishi, Y.; Tesana, S.; Metha, G.F.; Andersson, G.G. Suppression of phosphine-protected Au9 cluster agglomeration on SrTiO3 particles using a chromium hydroxide layer. Mater. Adv. 2022, 3, 3620–3630. [Google Scholar] [CrossRef]
- Smoluchowski, M.V. Drei vortrage uber diffusion, brownsche bewegung und koagulation von kolloidteilchen. Z. Fur Phys. 1916, 17, 557–585. [Google Scholar]
- Ostwald, W. Blocking of Ostwald ripening allowing long-term stabilization. Phys. Chem. 1901, 37, 385. [Google Scholar]
- Wilcoxon, J.P.; Provencio, P. Etching and Aging Effects in Nanosize Au Clusters Investigated Using High-Resolution Size-Exclusion Chromatography. J. Phys. Chem. B 2003, 107, 12949. [Google Scholar] [CrossRef]
- Moulder, J.F. Handbook of X-ray photoelectron spectroscopy: A reference book of standard data for use in x-ray photoelectron spectroscopy. In Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Waltham, MA, USA, 1992. [Google Scholar]
- Biesinger, M.C.; Brown, C.; Mycroft, J.R.; Davidson, R.D.; McIntyre, N.S. X-ray photoelectron spectroscopy studies of chromium compounds. Surf. Interface Anal. 2004, 36, 1550–1563. [Google Scholar] [CrossRef]
- Kawawaki, T.; Kataoka, Y.; Hirata, M.; Akinaga, Y.; Takahata, R.; Wakamatsu, K.; Fujiki, Y.; Kataoka, M.; Kikkawa, S.; Alotabi, A.S.; et al. Creation of High-Performance Heterogeneous Photocatalysts by Controlling Ligand Desorption and Particle Size of Gold Nanocluster. Angew. Chem. Int. Ed. 2021, 60, 21340–21350. [Google Scholar] [CrossRef] [PubMed]
- Madridejos, J.M.L.; Harada, T.; Falcinella, A.J.; Small, T.D.; Golovko, V.B.; Andersson, G.G.; Metha, G.F.; Kee, T.W. Optical Properties of the Atomically Precise C4 Core [Au9(PPh3)8]3+ Cluster Probed by Transient Absorption Spectroscopy and Time-Dependent Density Functional Theory. J. Phys. Chem. C 2021, 125, 2033–2044. [Google Scholar] [CrossRef]
- Krishnan, G.; Al Qahtani, H.S.; Li, J.; Yin, Y.; Eom, N.; Golovko, V.B.; Metha, G.F.; Andersson, G.G. Investigation of Ligand-Stabilized Gold Clusters on Defect-Rich Titania. J. Phys. Chem. C 2017, 121, 28007–28016. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotabi, A.S.; Yin, Y.; Redaa, A.; Tesana, S.; Metha, G.F.; Andersson, G.G. Effect of TiO2 Film Thickness on the Stability of Au9 Clusters with a CrOx Layer. Nanomaterials 2022, 12, 3218. https://doi.org/10.3390/nano12183218
Alotabi AS, Yin Y, Redaa A, Tesana S, Metha GF, Andersson GG. Effect of TiO2 Film Thickness on the Stability of Au9 Clusters with a CrOx Layer. Nanomaterials. 2022; 12(18):3218. https://doi.org/10.3390/nano12183218
Chicago/Turabian StyleAlotabi, Abdulrahman S., Yanting Yin, Ahmad Redaa, Siriluck Tesana, Gregory F. Metha, and Gunther G. Andersson. 2022. "Effect of TiO2 Film Thickness on the Stability of Au9 Clusters with a CrOx Layer" Nanomaterials 12, no. 18: 3218. https://doi.org/10.3390/nano12183218