Sodium Pre-Intercalation-Based Na3-δ-MnO2@CC for High-Performance Aqueous Asymmetric Supercapacitor: Joint Experimental and DFT Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Treatment of Carbon Cloth (CC)
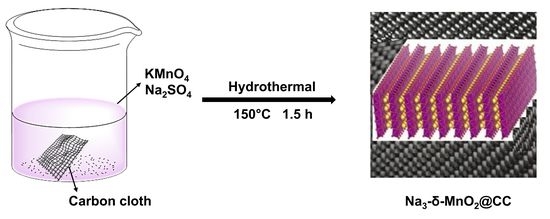
2.2. Synthesis of Na3-MnO2
3. Results
4. Computational Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, Z.; Miao, L.; Ruhlmann, L.; Lv, Y.; Zhu, D.; Li, L.; Gan, L.; Liu, M. Self-assembled carbon superstructures achieving ultra-stable and fast proton-coupled charge storage kinetics. Adv. Mater. 2021, 33, 2104148. [Google Scholar] [CrossRef] [PubMed]
- Muzaffar, A.; Basheer Ahamed, M.; Deshmukh, K. Nature-Inspired Electrodes for Flexible Supercapacitors. In Flexible Supercapacitor Nanoarchitectonics; Wiley: Hoboken, NJ, USA, 2021; pp. 549–573. [Google Scholar] [CrossRef]
- Shi, Z.; Sun, G.; Yuan, R.; Chen, W.; Wang, Z.; Zhang, L.; Zhan, K.; Zhu, M.; Yang, J.; Zhao, B. Scalable fabrication of NiCo2O4/reduced graphene oxide composites by ultrasonic spray as binder-free electrodes for supercapacitors with ultralong lifetime. J. Mater. Sci. Technol. 2022, 99, 260–269. [Google Scholar] [CrossRef]
- Zarshad, N.; Wu, J.; Rahman, A.U.; Ali, A.; Qiu, C.; Aisha, R.; Gao, R.; Xie, Y.; Ni, H. Binder and conductive agent-free electrode for the excellent aqueous asymmetrical supercapacitor. Solid State Sci. 2021, 112, 106530. [Google Scholar] [CrossRef]
- Sun, G.; Ren, H.; Shi, Z.; Zhang, L.; Wang, Z.; Zhan, K.; Yan, Y.; Yang, J.; Zhao, B. V2O5/vertically-aligned carbon nanotubes as negative electrode for asymmetric supercapacitor in neutral aqueous electrolyte. J. Colloid Interface Sci. 2021, 588, 847–856. [Google Scholar] [CrossRef]
- Salanne, M.; Rotenberg, B.; Naoi, K.; Kaneko, K.; Taberna, P.-L.; Grey, C.P.; Dunn, B.; Simon, P. Efficient storage mechanisms for building better supercapacitors. Nat. Energy 2016, 1, 16070. [Google Scholar] [CrossRef]
- Choi, C.; Ashby, D.S.; Butts, D.M.; DeBlock, R.H.; Wei, Q.; Lau, J.; Dunn, B. Achieving high energy density and high power density with pseudocapacitive materials. Nat. Rev. Mater. 2020, 5, 5–19. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, K.; Chen, Y. Wide Voltage Aqueous Asymmetric Supercapacitors: Advances, Strategies, and Challenges. Adv. Funct. Mater. 2021, 32, 2108107. [Google Scholar] [CrossRef]
- Rahman, A.U.; Zarshad, N.; Wu, J.; Faiz, F.; Raziq, F.; Ali, A.; Li, G.; Ni, H. Fabrication of Ag-doped MnO2 nanosheets@carbon cloth for energy storage device. Mater. Sci. Eng. B 2021, 269, 115150. [Google Scholar] [CrossRef]
- Zarshad, N.; Rahman, A.U.; Wu, J.; Ali, A.; Raziq, F.; Han, L.; Wang, P.; Li, G.; Ni, H. Enhanced energy density and wide potential window for K incorporated MnO2@carbon cloth supercapacitor. Chem. Eng. J. 2021, 415, 128967. [Google Scholar] [CrossRef]
- Zarshad, N.; Wu, J.; Rahman, A.U.; Tariq, M.; Ali, A.; Ni, H. MnO2 nanospheres electrode composed of low crystalline ultra-thin nanosheets for high performance and high rate supercapacitors. Mater. Sci. Eng. B 2020, 259, 114610. [Google Scholar] [CrossRef]
- Zarshad, N.; Wu, J.; Rahman, A.U.; Yu, H.; Ali, A.; Ni, H. Green and environmental-friendly method to synthesize template free nano grass-flower hierarchical manganese dioxide for high performance supercapacitor. Solid State Sci. 2020, 106, 106138. [Google Scholar] [CrossRef]
- Ghosh, S.K. Diversity in the family of manganese oxides at the nanoscale: From fundamentals to applications. ACS Omega 2020, 5, 25493–25504. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Kosasang, S.; Krittayavathananon, A.; Phattharasupakun, N.; Sethuraman, S.; Sawangphruk, M. Effect of intercalated alkali ions in layered manganese oxide nanosheets as neutral electrochemical capacitors. Chem. Commun. 2019, 55, 1213–1216. [Google Scholar] [CrossRef]
- Du, W.; Miao, L.; Song, Z.; Zheng, X.; Lv, Y.; Zhu, D.; Gan, L.; Liu, M. Kinetics-driven design of 3D VN/MXene composite structure for superior zinc storage and charge transfer. J. Power Sources 2022, 536, 231512. [Google Scholar] [CrossRef]
- He, S.; Mo, Z.; Shuai, C.; Liu, W.; Yue, R.; Liu, G.; Pei, H.; Chen, Y.; Liu, N.; Guo, R. Pre-intercalation δ-MnO2 Zinc-ion hybrid supercapacitor with high energy storage and Ultra-long cycle life. Appl. Surf. Sci. 2022, 577, 151904. [Google Scholar] [CrossRef]
- Vedpathak, A.S.; Desai, M.A.; Bhagwat, S.; Sartale, S.D. Green strategy for the synthesis of K+ pre-inserted MnO2/rGO and its electrochemical conversion to Na-MnO2/rGO for high-performance supercapacitors. Energy Fuels 2022, 36, 4596–4608. [Google Scholar] [CrossRef]
- Chen, Q.; Jin, J.; Kou, Z.; Liao, C.; Liu, Z.; Zhou, L.; Wang, J.; Mai, L.J.S. Zn2+ pre-intercalation stabilizes the tunnel structure of MnO2 nanowires and enables zinc-ion hybrid supercapacitor of battery-level energy density. Small 2020, 16, 2000091. [Google Scholar] [CrossRef]
- Duan, H.; Song, Z.; Miao, L.; Li, L.; Zhu, D.; Gan, L.; Liu, M. Unraveling the role of solvent–precursor interaction in fabricating heteroatomic carbon cathode for high-energy-density Zn-ion storage. J. Mater. Chem. A 2022, 10, 9837–9847. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Y.-C.; Huang, L.; Liu, K.; Duployer, B.; Rozier, P.; Taberna, P.-L.; Simon, P. Alkali ions pre-intercalated layered MnO2 nanosheet for zinc-ions storage. Adv. Energy Mater. 2021, 11, 2101287. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhan, C.; He, K.; Chen, H.; Yao, W.; Sharifi-Asl, S.; Song, B.; Yang, Z.; Nie, A.; Luo, X.; et al. The influence of large cations on the electrochemical properties of tunnel-structured metal oxides. Nat. Commun. 2016, 7, 13374. [Google Scholar] [CrossRef]
- Zhao, R.; Zhang, L.; Wang, C.; Yin, L. Tetramethyl ammonium cation intercalated layered birnessite manganese dioxide for high-performance intercalation pseudocapacitor. J. Power Sources 2017, 353, 77–84. [Google Scholar] [CrossRef]
- Wang, X.; Hu, P.; Niu, C.; Meng, J.; Xu, X.; Wei, X.; Tang, C.; Luo, W.; Zhou, L.; An, Q.; et al. New-type K0.7Fe0.5Mn0.5O2 cathode with an expanded and stabilized interlayer structure for high-capacity sodium-ion batteries. Nano Energy 2017, 35, 71–78. [Google Scholar] [CrossRef]
- Kang, K.; Ceder, G. Factors that affect Li mobility in layered lithium transition metal oxides. Phys. Rev. B 2006, 74, 094105. [Google Scholar] [CrossRef]
- Nam, K.W.; Kim, S.; Yang, E.; Jung, Y.; Levi, E.; Aurbach, D.; Choi, J.W. Critical role of crystal water for a layered cathode material in sodium ion batteries. Chem. Mater. 2015, 27, 3721–3725. [Google Scholar] [CrossRef]
- Frey, N.C.; Byles, B.W.; Kumar, H.; Er, D.; Pomerantseva, E.; Shenoy, V.B. Prediction of optimal structural water concentration for maximized performance in tunnel manganese oxide electrodes. Phys. Chem. Chem. Phys. 2018, 20, 9480–9487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, J.; Ahmed Shifa, T.; Wang, D.; Wu, X.; Cui, Y. Hierarchical MnO2/activated carbon cloth electrode prepared by synchronized electrochemical activation and oxidation for flexible asymmetric supercapacitors. Chem. Eng. J. 2019, 372, 1047–1055. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, S.; Cui, W.; Lv, J.; Geng, Y.; Li, H.; Deng, J. Interconnected network of ultrafine MnO2 nanowires on carbon cloth with weed-like morphology for high-performance supercapacitor electrodes. Electrochim. Acta 2018, 268, 340–346. [Google Scholar] [CrossRef]
- Guo, C.; Liu, H.; Li, J.; Hou, Z.; Liang, J.; Zhou, J.; Zhu, Y.; Qian, Y. Ultrathin δ-MnO2 nanosheets as cathode for aqueous rechargeable zinc ion battery. Electrochim. Acta 2019, 304, 370–377. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Wang, J.; Lee, P.S. Sulfidation of NiMn-layered double hydroxides/graphene oxide composites toward supercapacitor electrodes with enhanced performance. Adv. Energy Mater. 2016, 6, 1501745. [Google Scholar] [CrossRef]
- Lan, B.; Huang, S.; Ye, C.; Qin, Q.; Yan, J.; Wu, Y. Enhanced electrochemical performance of Sn-doped MnO2 and study on morphology evolution. J. Alloys Compd. 2019, 788, 302–310. [Google Scholar] [CrossRef]
- Long, X.; Tian, L.; Wang, J.; Zhang, L.; Chen, Y.; Emin, A.; Wang, X.; Xie, W.; Liu, D.; Fu, Y.; et al. Interconnected δ-MnO2 nanosheets anchored on activated carbon cloth as flexible electrode for high-performance aqueous asymmetric supercapacitors. J. Electroanal. Chem. 2020, 877, 114656. [Google Scholar] [CrossRef]
- Nasser, R.; Zhang, G.-F.; Song, J.-M. Facile and low-cost synthesis of cobalt-doped MnO2 decorated with graphene oxide for high performance 2.3 V aqueous asymmetric supercapacitors. Electrochim. Acta 2020, 345, 136198. [Google Scholar] [CrossRef]
- Gu, Y.-J.; Wen, W.; Wu, J.-M. Wide potential window TiO2@carbon cloth and high capacitance MnO2@carbon cloth for the construction of a 2.6 V high-performance aqueous asymmetric supercapacitor. J. Power Sources 2020, 469, 228425. [Google Scholar] [CrossRef]
- Zong, Q.; Zhang, Q.; Mei, X.; Li, Q.; Zhou, Z.; Li, D.; Chen, M.; Shi, F.; Sun, J.; Yao, Y.; et al. Facile synthesis of na-doped MnO2 nanosheets on carbon nanotube fibers for ultrahigh-energy-density all-solid-state wearable asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 37233–37241. [Google Scholar] [CrossRef] [PubMed]
- Sehrawat, D.; Rawal, A.; Cheong, S.; Avdeev, M.; Ling, C.D.; Kimpton, J.A.; Sharma, N. Alkali metal-modified P2 NaxMnO2: Crystal structure and application in sodium-ion batteries. Inorg. Chem. 2020, 59, 12143–12155. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, M.; Ma, X.; Li, L.; Zhou, X.; Zhang, Z. Asymmetric supercapacitors by integrating high content Na+/K+-inserted MnO2 nanosheets and layered Ti3C2Tx paper. Electrochim. Acta 2020, 332, 135497. [Google Scholar] [CrossRef]
- Kulkarni, S.; Puthusseri, D.; Thakur, S.; Banpurkar, A.; Patil, S. Hausmannite manganese oxide cathodes for supercapacitors: Surface wettability and electrochemical properties. Electrochim. Acta 2017, 231, 460–467. [Google Scholar] [CrossRef]
- Singu, B.S.; Yoon, K.R. Exfoliated graphene-manganese oxide nanocomposite electrode materials for supercapacitor. J. Alloys Compd. 2019, 770, 1189–1199. [Google Scholar] [CrossRef]
- Pendashteh, A.; Senokos, E.; Palma, J.; Anderson, M.; Vilatela, J.J.; Marcilla, R. Manganese dioxide decoration of macroscopic carbon nanotube fibers: From high-performance liquid-based to all-solid-state supercapacitors. J. Power Sources 2017, 372, 64–73. [Google Scholar] [CrossRef]
- Shao, J.; Zhou, X.; Liu, Q.; Zou, R.; Li, W.; Yang, J.; Hu, J. Mechanism analysis of the capacitance contributions and ultralong cycling-stability of the isomorphous MnO2@MnO2 core/shell nanostructures for supercapacitors. J. Mater. Chem. A 2015, 3, 6168–6176. [Google Scholar] [CrossRef]
- Jabeen, N.; Xia, Q.; Savilov, S.V.; Aldoshin, S.M.; Yu, Y.; Xia, H. Enhanced pseudocapacitive performance of α-MnO2 by cation preinsertion. ACS Appl. Mater. Interfaces 2016, 8, 33732–33740. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Gao, M.; Yu, S.; Feng, M.; Liu, S.; Fu, J. MnO2 nanosheets grown on N and P co-doped hollow carbon microspheres for high performance asymmetric supercapacitor. Electrochim. Acta 2020, 354, 136681. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Wang, D.; Zhao, J.; Zhang, H. Snowflake-like core-shell α-MnO2@δ-MnO2 for high performance asymmetric supercapacitor. Electrochim. Acta 2017, 251, 344–354. [Google Scholar] [CrossRef]
- Peng, H.; Fan, H.; Sui, J.; Wang, C.; Zhang, W.; Wang, W. Sodium in situ intercalated ultrathin δ-MnO2 flakes electrode with enhanced intercalation capacitive performance for asymmetric supercapacitors. ChemistrySelect 2020, 5, 869–874. [Google Scholar] [CrossRef]
- Jiang, L.; Dong, M.; Dou, Y.; Chen, S.; Liu, P.; Yin, H.; Zhao, H. Manganese oxides transformed from orthorhombic phase to birnessite with enhanced electrochemical performance as supercapacitor electrodes. J. Mater. Chem. A 2020, 8, 3746–3753. [Google Scholar] [CrossRef]
- Patil, S.J.; Chodankar, N.R.; Han, Y.-K.; Lee, D.W. Carbon alternative pseudocapacitive V2O5 nanobricks and δ-MnO2 nanoflakes @ α-MnO2 nanowires hetero-phase for high-energy pseudocapacitor. J. Power Sources 2020, 453, 227766. [Google Scholar] [CrossRef]
- Chen, Y.; Jing, C.; Fu, X.; Shen, M.; Cao, T.; Huo, W.; Liu, X.; Yao, H.-C.; Zhang, Y.; Yao, K.X. In-situ fabricating MnO2 and its derived FeOOH nanostructures on mesoporous carbon towards high-performance asymmetric supercapacitor. Appl. Surf. Sci. 2020, 503, 144123. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, Y.; Yan, Y.; Wei, B.; Qiao, K.; Zhu, B.; Cai, X.; Chou, T.-W. Tunable synthesis of biomass-based hierarchical porous carbon scaffold@MnO2 nanohybrids for asymmetric supercapacitor. Chem. Eng. J. 2020, 393, 121214. [Google Scholar] [CrossRef]
- Tanggarnjanavalukul, C.; Phattharasupakun, N.; Kongpatpanich, K.; Sawangphruk, M. Charge storage performances and mechanisms of MnO2 nanospheres, nanorods, nanotubes and nanosheets. Nanoscale 2017, 9, 13630–13639. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, G.; Ko, Y.H.; Cha, S.M.; Im, S.H.; Yu, J.S. A facile one-step approach to hierarchically assembled core–shell-like MnO2@MnO2 nanoarchitectures on carbon fibers: An efficient and flexible electrode material to enhance energy storage. Nano Res. 2016, 9, 1507–1522. [Google Scholar] [CrossRef]
- Guo, Y.; Li, L.; Song, L.; Wu, M.; Gao, Y.; Chen, J.; Mao, C.; Song, J.; Niu, H. Co2+ induced phase transformation from δ- to α-MnO2 and their hierarchical α-MnO2@δ-MnO2 nanostructures for efficient asymmetric supercapacitors. J. Mater. Chem. A 2019, 7, 12661–12668. [Google Scholar] [CrossRef]
- Rogier, C.; Pognon, G.; Bondavalli, P.; Galindo, C.; Nguyen, G.T.M.; Vancaeyzeele, C.; Aubert, P.-H. Electrodeposition of MnO2 on spray-coated nanostructured carbon framework as high performance material for energy storage. Surf. Coat. Technol. 2020, 384, 125310. [Google Scholar] [CrossRef]
- Peng, H.; Fan, H.; Yang, C.; Tian, Y.; Wang, C.; Sui, J. Ultrathin δ-MnO2 nanoflakes with Na+ intercalation as a high-capacity cathode for aqueous zinc-ion batteries. RSC Adv. 2020, 10, 17702–17712. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Niu, H.; Qin, F.; Guo, Z.; Wang, J.; Ni, G.; Zuo, P.; Qu, S.; Shen, W. MnO2 doped carbon nanosheets prepared from coal tar pitch for advanced asymmetric supercapacitor. Electrochim. Acta 2020, 354, 136667. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, H.; Zhu, H.; Xu, Z.; Liu, R.; Chen, J.; Song, Q.; Song, X.; Wu, J.; Zhang, C.; et al. Graphene-wrapped MnO2 achieved by ultrasonic-assisted synthesis applicable for hybrid high-energy supercapacitors. Vacuum 2020, 176, 109315. [Google Scholar] [CrossRef]
- Feng, W.; Liu, G.; Wang, P.; Zhou, J.; Gu, L.; Chen, L.; Li, X.; Dan, Y.; Cheng, X. Template synthesis of a heterostructured MnO2@SnO2 hollow sphere composite for high asymmetric supercapacitor performance. ACS Appl. Energy Mater. 2020, 3, 7284–7293. [Google Scholar] [CrossRef]
- Li, D.; Lin, J.; Lu, Y.; Huang, Y.; He, X.; Yu, C.; Zhang, J.; Tang, C. MnO2 nanosheets grown on N-doped agaric-derived three-dimensional porous carbon for asymmetric supercapacitors. J. Alloys Compd. 2020, 815, 152344. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Dou, J.; Zhang, P. Construction of MnO2@NH4MnF3 core-shell nanorods for asymmetric supercapacitor. Electrochim. Acta 2020, 347, 136257. [Google Scholar] [CrossRef]
- Tan, Y.; Yang, C.; Qian, W.; Teng, C. Flower-like MnO2 on layered carbon derived from sisal hemp for asymmetric supercapacitor with enhanced energy density. J. Alloys Compd. 2020, 826, 154133. [Google Scholar] [CrossRef]
- Zarshad, N.; Wu, J.; Rahman, A.U.; Ni, H. Fe-MnO2 core-shell heterostructure for high-performance aqueous asymmetrical supercapacitor. J. Electroanal. Chem. 2020, 871, 114266. [Google Scholar] [CrossRef]
- Fu, Y.; Gao, X.; Zha, D.; Zhu, J.; Ouyang, X.; Wang, X. Yolk–shell-structured MnO2 microspheres with oxygen vacancies for high-performance supercapacitors. J. Mater. Chem. A 2018, 6, 1601–1611. [Google Scholar] [CrossRef]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+ U study. Phys. Rev. B 1998, 57, 1505. [Google Scholar] [CrossRef]
- Kitchaev, D.A.; Peng, H.; Liu, Y.; Sun, J.; Perdew, J.P.; Ceder, G. Energetics of MnO 2 polymorphs in density functional theory. Phys. Rev. B 2016, 93, 045132. [Google Scholar] [CrossRef]
- Franchini, C.; Podloucky, R.; Paier, J.; Marsman, M.; Kresse, G. Ground-state properties of multivalent manganese oxides: Density functional and hybrid density functional calculations. Phys. Rev. B 2007, 75, 195128. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, A.U.; Zarshad, N.; Jianghua, W.; Shah, M.; Ullah, S.; Li, G.; Tariq, M.; Ali, A. Sodium Pre-Intercalation-Based Na3-δ-MnO2@CC for High-Performance Aqueous Asymmetric Supercapacitor: Joint Experimental and DFT Study. Nanomaterials 2022, 12, 2856. https://doi.org/10.3390/nano12162856
Rahman AU, Zarshad N, Jianghua W, Shah M, Ullah S, Li G, Tariq M, Ali A. Sodium Pre-Intercalation-Based Na3-δ-MnO2@CC for High-Performance Aqueous Asymmetric Supercapacitor: Joint Experimental and DFT Study. Nanomaterials. 2022; 12(16):2856. https://doi.org/10.3390/nano12162856
Chicago/Turabian StyleRahman, Anis Ur, Nighat Zarshad, Wu Jianghua, Muslim Shah, Sana Ullah, Guigen Li, Muhammad Tariq, and Asad Ali. 2022. "Sodium Pre-Intercalation-Based Na3-δ-MnO2@CC for High-Performance Aqueous Asymmetric Supercapacitor: Joint Experimental and DFT Study" Nanomaterials 12, no. 16: 2856. https://doi.org/10.3390/nano12162856