Natural Diatomite Supported Zirconium-Doped TiO2 with Tailoring Band Structure for Enhanced Visible-Light Photocatalytic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Zr Doped TiO/Diatomite Photocatalysts
2.3. Equipment and Characterizations
2.4. Evaluation of Photocatalytic Activity
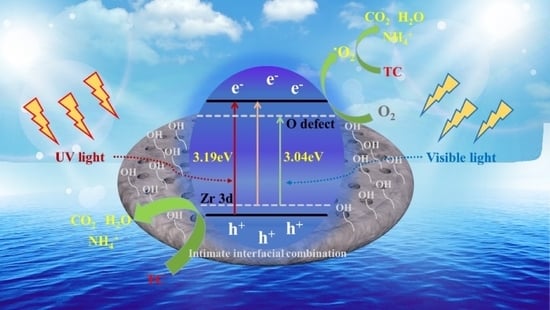
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sample | k (min) | R |
|---|---|---|
| T | 0.178 × 10 | 0.9889 |
| TD | 0.952 × 10 | 0.9854 |
| ZrT | 1.179 × 10 | 0.9849 |
| 1%ZrTD | 1.294 × 10 | 0.9855 |
| 2%ZrTD | 1.733 × 10 | 0.9846 |
| 3%ZrTD | 1.901 × 10 | 0.9889 |
| 4%ZrTD | 1.482 × 10 | 0.9825 |
| Photocatalysts | Photocatalyst Concentration (g·L) | TC Concentration (mg·L) | Removal Time (min) | Removal Rate (%) | Reference |
|---|---|---|---|---|---|
| 3%ZrTD | 1.0 | 20 | 120 | 93.5 | Present work |
| P25 | 0.1 | 10 | 90 | 29.1 | [42] |
| BiWO | 1.0 | 20 | 120 | 48.4 | [6] |
| PO-BiWO/polyimide | 1.0 | 20 | 16 | 65.1 | [6] |
| MoS | 0.2 | 20 | 180 | 48.5 | [43] |
| MoS@Z-5 | 0.5 | 10 | 180 | 87.2 | [43] |
| CuInS/BiMoO | 0.6 | 15 | 120 | 84.7 | [44] |
| Ag/g-CN-Ag-AgPO | 1.0 | 10 | 100 | 90.2 | [45] |
| ZnO | 0.1 | 50 | 240 | 83.7 | [46] |
| MIL-100(Fe) | 0.7 | 10 | 120 | 90.3 | [47] |
| Zeolite@ZIF-67@PMS | - | 50 | 60 | 93.7 | [48] |
References
- Li, C.G.; Tian, Q.; Zhang, Y.L.; Li, Y.Y.; Yang, X.M.; Zheng, H.; Chen, L.Y.; Li, F.M. Sequential combination of photocatalysis and microalgae technology for promoting the degradation and detoxification of typical antibiotics. Water Res. 2022, 210, 117985. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Shu, Z.; Chen, Z.N.; Su, J.X.; Liu, C.G. Iodide ions enhancing sulfamerazine degradation by horseradish peroxidase/H2O2: Degradation products, degradation mechanism and toxicity assessment. J. Clean Prod. 2022, 337, 130489. [Google Scholar] [CrossRef]
- Wang, M.; Lu, G.H.; Jiang, R.R.; Dang, T.J.; Liu, J.C. Degradation and detoxification of broad-spectrum antibiotics by small molecular intercalated BiOCl under visible light. J. Colloid Interface Sci. 2022, 622, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Q.; Zheng, Z.K.; Wang, H.; Chen, Y.W.; Luo, C.H.; Liang, D.J.; Hu, B.W.; Qiu, R.L.; Yan, K. 3D hierarchical H2-reduced Mn-doped CeO2 microflowers assembled from nanotubes as a high-performance Fenton-like photocatalyst for tetracycline antibiotics degradation. Appl. Catal. B-Environ. 2020, 277, 119171. [Google Scholar] [CrossRef]
- Ahmed, M.J. Adsorption of quinolone, tetracycline, and penicillin antibiotics from aqueous solution using activated carbons: Review. Environ. Toxicol. Pharmacol. 2017, 50, 1–10. [Google Scholar] [CrossRef]
- Gao, X.; Niu, J.; Wang, Y.F.; Ji, Y.; Zhang, Y.L. Solar photocatalytic abatement of tetracycline over phosphate oxoanion decorated Bi2WO2/polyimide composites. J. Hazard. Mater. 2021, 403, 123860. [Google Scholar] [CrossRef]
- Zhong, S.F.; Yang, B.; Xiong, Q.; Cai, W.W.; Lan, Z.G.; Ying, G.G. Hydrolytic transformation mechanism of tetracycline antibiotics: Reaction kinetics, products identification and determination in WWTPs. Ecotox. Environ. Safe 2022, 229, 113063. [Google Scholar] [CrossRef]
- Tyagi, N.; Kumar, A. Evaluation of recreational risks due to exposure of antibiotic-resistance bacteria from environmental water: A proposed framework. J. Environ. Manag. 2021, 279, 111626. [Google Scholar] [CrossRef]
- Wang, T.; Xue, L.; Liu, Y.H.; Fang, T.; Zhang, L.; Xing, B.S. Insight into the significant contribution of intrinsic defects of carbon-based materials for the efficient removal of tetracycline antibiotics. Chem. Eng. J. 2022, 435, 134822. [Google Scholar] [CrossRef]
- Wang, W.; Gao, M.; Cao, M.B.; Liu, X.; Yang, H.B.; Li, Y.S. A series of novel carbohydrate-based carbon adsorbents were synthesized by self-propagating combustion for tetracycline removal. Bioresour. Technol. 2021, 332, 125059. [Google Scholar] [CrossRef]
- Yue, R.Y.; Sun, X.F. A Self-cleaning, catalytic titanium carbide (MXene) membrane for efficient tetracycline degradation through peroxymonosulfate activation: Performance evaluation and mechanism study. Sep. Purif. Technol. 2021, 279, 119796. [Google Scholar] [CrossRef]
- Mohsin, M.K.; Mohammed, A.A. Catalytic ozonation for removal of antibiotic oxy-tetracycline using zinc oxide nanoparticles. Appl. Water Sci. 2021, 11, 9. [Google Scholar] [CrossRef]
- Ma, C.B.; Zhang, Y.Q. Spinel CuxCo1-xMn2O4 electrode for effectively cleaning organic wastewater via electrocatalytic oxidation. Sep. Purif. Technol. 2022, 435, 134822. [Google Scholar] [CrossRef]
- Wang, C.C.; Cai, M.J.; Liu, Y.P.; Yang, F.; Zhang, H.Q.; Liu, J.S.; Li, S.J. Facile construction of novel organic-inorganic tetra (4-carboxyphenyl) porphyrin/Bi2MoO6 heterojunction for tetracycline degradation: Performance, degradation pathways, intermediate toxicity analysis and mechanism insight. J. Colloid Interface Sci. 2022, 605, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Fang, X.; Wan, Y.C.; Zhan, J.; Wang, Z.J.; Liu, H. Visible-light-induced NiCo2O4@Co3O4 core/shell heterojunction photocatalysts for efficient removal of organic dyes. J. Cent. South Univ. 2021, 28, 3040–3049. [Google Scholar] [CrossRef]
- Tan, X.; Zhou, S.; Tao, H.J.; Wang, W.Y.; Wan, Q.W.; Zhang, K.C. Influence of Ag on photocatalytic performance of Ag/ZnO nanosheet photocatalysts. J. Cent. South Univ. 2019, 26, 2011–2018. [Google Scholar] [CrossRef]
- Juma, A.; Lemoine, P.; Simpson, A.B.J.; Murray, J.; O’Hagan, B.M.G.; Naughton, P.J.; Dooley, J.G. Banat, I.M. Microscopic investigation of the combined use of antibiotics and biosurfactants on methicillin resistantstaphylococcus aureus. Front. Microbiol. 2020, 11, 1477. [Google Scholar] [CrossRef]
- Li, Z.H.; Yuan, L.; Wang, L.; Liu, Q.H.; Sheng, G.P. Coexistence of silver ion and tetracycline at environmentally relevant concentrations greatly enhanced antibiotic resistance gene development in activated sludge bioreactor. J. Hazard. Mater. 2022, 423, 127088. [Google Scholar] [CrossRef]
- Liu, H.D.; Xu, G.R.; Li, G.B. Preparation of porous biochar based on pharmaceutical sludge activated by NaOH and its application in the adsorption of tetracycline. J. Colloid Interface Sci. 2019, 26, 2011–2018. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.L.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Tan, Y.G.; Shu, Z.; Zhou, J.; Li, T.T.; Wang, W.B.; Zhao, Z.L. One-step synthesis of nanostructured g-C3N4/TiO2 composite for highly enhanced visible-light photocatalytic H2 evolution. Appl. Catal. B-Environ. 2018, 230, 260–268. [Google Scholar] [CrossRef]
- Naldoni, A.; Altomare, M.; Zoppellaro, G.; Liu, N.; Kment, S.; Zboril, R.; Schmuki, P. Photocatalysis with Reduced TiO2: From Black TiO2 to Cocatalyst-Free Hydrogen Production. ACS Catal. 2019, 9, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Zhou, Y.; Huang, C.; Huang, Q.; Chen, Y.; Xu, H.; Shen, S. Impact of Zr-doped TiO2 photocatalyst on formaldehyde degradation by Na addition. Ind. Eng. Chem. Res. 2018, 57, 14044–14051. [Google Scholar] [CrossRef]
- Pouretedal, H.R. Visible photocatalytic activity of co-doped TiO2/Zr,N nanoparticles in wastewater treatment of nitrotoluene samples. J. Alloy. Compd. 2018, 735, 2507–2511. [Google Scholar] [CrossRef]
- Yuan, F.; Yang, R.; Li, C.; Tan, Y.; Zhang, X.; Zheng, S.; Sun, Z. Enhanced visible-light degradation performance toward gaseous formaldehyde using oxygen vacancy-rich TiO2-x/TiO2 supported by natural diatomite. Build. Environ. 2022, 219, 109216. [Google Scholar] [CrossRef]
- He, F.; Meng, A.Y.; Cheng, B.; Ho, W.K.; Yu, J.G. Enhanced photocatalytic H-2-production activity of WO3/TiO2 step-scheme heterojunction by graphene modification. Chin. J. Catal. 2020, 41, 9–20. [Google Scholar] [CrossRef]
- Chen, D.J.; Cheng, Y.L.; Zhou, N.; Chen, P.; Wang, Y.P.; Li, K.; Huo, S.H.; Cheng, P.F.; Peng, P.; Zhang, R.C.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Yuan, F.; Sun, Z.; Li, C.; Tan, Y.; Zhang, X.; Zheng, S. Multi-component design and in-situ synthesis of visible-light-driven SnO2/g-C3N4/diatomite composite for high-efficient photoreduction of Cr(VI) with the aid of citric acid. J. Hazard. Mater. 2020, 396, 122694. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Chen, T.; Tan, Y.; Liu, X.; Yuan, F.; Zheng, S.; Sun, Z. Enhanced visible-light-assisted peroxymonosulfate activation over MnFe2O4 modified g-C3N4/diatomite composite for bisphenol A degradation. Int. J. Min. Sci. Technol. 2021, 31, 1169–1179. [Google Scholar] [CrossRef]
- Yuan, F.; Li, C.; Yang, R.; Tan, Y.; Ma, R.; Zhang, X.; Zheng, S.; Sun, Z. High-efficient mineralization of formaldehyde by three-dimensional “PIZZA”-like bismuth molybdate-titania/diatomite composite. J. Colloid Interface Sci. 2022, 624, 713–724. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, X.; Dong, X.; Liu, X.; Tan, Y.; Yuan, F.; Zheng, S.; Li, C. Hierarchical assembly of highly efficient visible-light-driven Ag/g-C3N4/kaolinite composite photocatalyst for the degradation of ibuprofen. J. Mater. 2020, 6, 582–592. [Google Scholar] [CrossRef]
- Zhang, X.W.; Liu, Y.Y.; Li, C.Q.; Tian, L.; Yuan, F.; Zheng, S.L.; Sun, Z.M. Fast and lasting electron transfer between gamma-FeOOH and g-C3N4/kaolinite containing N vacancies for enhanced visible-light-assisted peroxymonosulfate activation. Chem. Eng. J. 2022, 429, 132347. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Zheng, S.; Sun, Z. Photocatalysis coupled with persulfate oxidation over nitrogen vacancies modified g-C3N4/kaolinite composite for highly efficient removal of bisphenol A. J. Chin. Ceram. Soc. 2021, 49, 1337–1346. [Google Scholar]
- Wu, A.C.; Wang, D.X.; Wei, C.; Zhang, X.D.; Liu, Z.S.; Feng, P.Z.; Ou, X.M.; Qiang, Y.H.; Garcia, H.; Niu, J. A comparative photocatalytic study of TiO2 loaded on three natural clays with different morphologies. Appl. Clay Sci. 2019, 183, 105352. [Google Scholar] [CrossRef]
- Di, Y.; Yuan, F.; Ning, X.; Jia, H.; Liu, Y.; Zhang, X.; Li, C.; Zheng, S.; Sun, Z. Functionalization of diatomite with glycine and amino silane for formaldehyde removal. Int. J. Miner. Metall. Mater. 2022, 29, 356–367. [Google Scholar] [CrossRef]
- Cheng, Z.Y.Y.; Zhu, Y.M.; Li, Y.J.; Butt, S. Experimental and MD simulation of 3-dodecyloxypropanamine and 3-tetradecyloxypropylamine adsorbed onto quartz (101) surface. Int. J. Miner. Metall. Mater. 2021, 31, 1033–1042. [Google Scholar]
- Huang, J.; Dou, L.; Li, J.; Zhong, J.; Li, M.; Wang, T. Excellent visible light responsive photocatalytic behavior of N-doped TiO2 toward decontamination of organic pollutants. J. Hazard. Mater. 2021, 403, 123857. [Google Scholar] [CrossRef]
- Yang, J.; Lin, Y.H.; Yang, X.D.; Ng, T.B.; Ye, X.Y.; Lin, J. Degradation of tetracycline by immobilized laccase and the proposed transformation pathway. J. Hazard. Mater. 2017, 322, 525–531. [Google Scholar] [CrossRef]
- Chen, H.L.; Peng, Y.P.; Chen, K.F.; Lai, C.H.; Lin, Y.C. Rapid synthesis of Ti-MCM-41 by microwave-assisted hydrothermal method towards photocatalytic degradation of oxytetracycline. J. Environ. Sci. 2016, 44, 76–87. [Google Scholar] [CrossRef]
- Zhu, X.D.; Wang, Y.J.; Sun, R.J.; Zhou, D.M. Photocatalytic degradation of tetracycline in aqueous solution by nanosized TiO2. Chemosphere 2013, 92, 925–932. [Google Scholar] [CrossRef]
- Wang, J.B.; Zhi, D.; Zhou, H.; He, X.W.; Zhang, D.Y. Evaluating tetracycline degradation pathway and intermediate toxicity during the electrochemical oxidation over a Ti/Ti4O7 anode. Water Res. 2018, 137, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.L.; Wei, L.X.; Ren, Z.Q.; Lu, J.F.; Ma, J. The facile fabrication of Z-scheme Bi2WO6-P25 heterojunction with enhanced photodegradation of antibiotics under visible light. J. Environ. Chem. Eng. 2021, 9, 106167. [Google Scholar] [CrossRef]
- Liu, J.F.; Lin, H.; Dong, Y.B.; He, Y.H.; Liu, C.J. MoS2 nanosheets loaded on collapsed structure zeolite as a hydrophilic and efficient photocatalyst for tetracycline degradation and synergistic mechanism. Chemosphere 2022, 287, 132211. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.R.; Wang, L.P.; Wei, X.; Alothman, Z.A.; Malgras, V.; Yamauchi, Y.; Kang, Y.Q.; Wang, M.Q.; Guan, W.S.; Xu, X.T. Albaqami, M.D. Direct Z-scheme CuInS2/Bi2MoO6 heterostructure for enhanced photocatalytic degradation of tetracycline under visible light. J. Hazard. Mater. 2021, 415, 125591. [Google Scholar] [CrossRef]
- Li, S.Y.; Zhang, M.; Qu, Z.H.; Cui, X.; Liu, Z.Y.; Piao, C.C.; Li, S.G.; Wang, J.; Song, Y.T. Fabrication of highly active Z-scheme Ag/g-C3N4-Ag-Ag3PO4 (110) photocatalyst photocatalyst for visible light photocatalytic degradation of levofloxacin with simultaneous hydrogen production. Chem. Eng. J. 2020, 382, 122394. [Google Scholar] [CrossRef]
- Chen, P.; Dong, N.N.; Zhang, J.J.; Wang, W.; Tan, F.T.; Wang, X.Y.; Qiao, X.L.; Wong, P.K. Investigation on visible-light photocatalytic performance and mechanism of zinc peroxide for tetracycline degradation and Escherichia coli inactivation. J. Colloid Interface Sci. 2022, 624, 137149. [Google Scholar] [CrossRef]
- Xu, W.G.; Xu, J.; Zhang, Q.Y.; Yun, Z.P.; Zuo, Q.S.; Wang, L.P. Study on visible light photocatalytic performance of MIL-100(Fe) modified by carbon nanodots. Environ. Sci. Pollut. Res. 2022, 29, 55069–55080. [Google Scholar] [CrossRef]
- Chen, D.Y.; Bai, Q.; Ma, T.T.; Jing, X.F.; Tian, Y.Y.; Zhao, R.; Zhu, G.S. Stable metal-organic framework fixing within zeolite beads for effectively static and continuous flow degradation of tetracycline by peroxymonosulfate activation. Chem. Eng. J. 2022, 435, 134916. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, F.; Li, C.; Zhang, X.; Yang, R.; Sun, Z. Natural Diatomite Supported Zirconium-Doped TiO2 with Tailoring Band Structure for Enhanced Visible-Light Photocatalytic Properties. Nanomaterials 2022, 12, 2827. https://doi.org/10.3390/nano12162827
Yuan F, Li C, Zhang X, Yang R, Sun Z. Natural Diatomite Supported Zirconium-Doped TiO2 with Tailoring Band Structure for Enhanced Visible-Light Photocatalytic Properties. Nanomaterials. 2022; 12(16):2827. https://doi.org/10.3390/nano12162827
Chicago/Turabian StyleYuan, Fang, Chunquan Li, Xiangwei Zhang, Renfeng Yang, and Zhiming Sun. 2022. "Natural Diatomite Supported Zirconium-Doped TiO2 with Tailoring Band Structure for Enhanced Visible-Light Photocatalytic Properties" Nanomaterials 12, no. 16: 2827. https://doi.org/10.3390/nano12162827