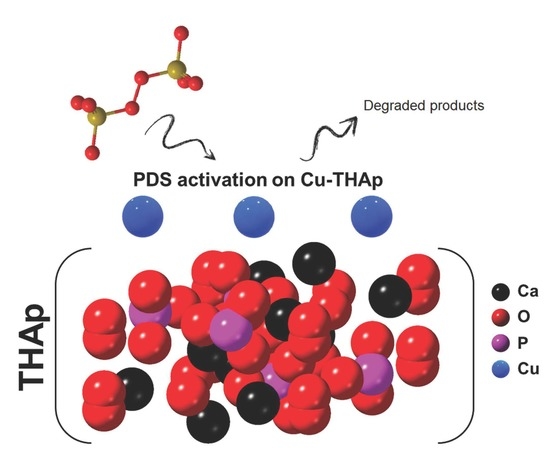
In Situ Formation of Copper Phosphate on Hydroxyapatite for Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Characterization
2.3. Synthesis
2.3.1. Synthesis of g-C3N4
2.3.2. Synthesis of g-C3N4-HAp (THAp)
2.3.3. Synthesis of Cu-Loaded THAp (Cu-THAp)
2.4. Catalytic RhB Degradation Experiments
3. Results and Discussion
3.1. Characterization of Synthesized Materials
3.2. PDS Activation for RhB Catalytic Degradation and Cu-THAp’s Reusability Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mohd Pu’ad, N.A.S.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doustkhah, E.; Najafi Zare, R.; Yamauchi, Y.; Taheri-Kafrani, A.; Mohtasham, H.; Esmat, M.; Ide, Y.; Fukata, N.; Rostamnia, S.; Sadeghi, M.H.; et al. Template-oriented synthesis of hydroxyapatite nanoplates for 3D bone printing. J. Mater. Chem. B 2019, 7, 7228–7234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, D.; Liu, S.; Dong, X.; Li, Y.; Zhang, H.; Yang, Z.; Su, Q.; Huang, W.; Zheng, W.; et al. Zirconia toughened hydroxyapatite biocomposite formed by a DLP 3D printing process for potential bone tissue engineering. Mater. Sci. Eng. C 2019, 105, 110054. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan-Wong, K.; Enax, J.; Meyer, F.; Ganss, B. The use of hydroxyapatite toothpaste to prevent dental caries. Odontology 2022, 110, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P.; Barrère, F.; Van Blitterswijk, C.A.; de Groot, K.; Layrolle, P. Biomimetic Hydroxyapatite Coating on Metal Implants. J. Am. Ceram. Soc. 2002, 85, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Maschmeyer, T.; Luque, R.; Selva, M. Upgrading of marine (fish and crustaceans) biowaste for high added-value molecules and bio(nano)-materials. Chem. Soc. Rev. 2020, 49, 4527–4563. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Pan, W.; Gu, T.; Bu, C.; Zhang, J.; Wang, X.; Liu, C.; Xie, H.; Piao, G. One-Pot Synthesis of a Highly Active and Stable Ni-Embedded Hydroxyapatite Catalyst for Syngas Production via Dry Reforming of Methane. Energy Fuels 2021, 35, 19568–19580. [Google Scholar] [CrossRef]
- El-Hosainy, H.; Mine, S.; Toyao, T.; Shimizu, K.-i.; Tsunoji, N.; Esmat, M.; Doustkhah, E.; El-Kemary, M.; Ide, Y. Layered silicate stabilises diiron to mimic UV-shielding TiO2 nanoparticle. Mater. Today Nano 2022, 19, 100227. [Google Scholar] [CrossRef]
- Esmat, M.; Doustkhah, E.; Abdelbar, M.; Tahawy, R.; El-Hosainy, H.; Abdelhameed, M.; Ide, Y.; Fukata, N. Structural Conversion of Cu-Titanate into Photoactive Plasmonic Cu-TiO2 for H2 Generation in Visible Light. ACS Sustain. Chem. Eng. 2022, 10, 4143–4151. [Google Scholar] [CrossRef]
- Pang, Y.; Kong, L.; Chen, D.; Yuvaraja, G.; Mehmood, S. Facilely synthesized cobalt doped hydroxyapatite as hydroxyl promoted peroxymonosulfate activator for degradation of Rhodamine B. J. Hazard. Mater. 2020, 384, 121447. [Google Scholar] [CrossRef]
- Jamal Sisi, A.; Fathinia, M.; Khataee, A.; Orooji, Y. Systematic activation of potassium peroxydisulfate with ZIF-8 via sono-assisted catalytic process: Mechanism and ecotoxicological analysis. J. Mol. Liq. 2020, 308, 113018. [Google Scholar] [CrossRef]
- He, C.; Liu, Z.; Wu, J.; Pan, X.; Fang, Z.; Li, J.; Bryan, B.A. Future global urban water scarcity and potential solutions. Nat. Commun. 2021, 12, 4667. [Google Scholar] [CrossRef] [PubMed]
- Ricart, S.; Villar-Navascués, R.A.; Hernández-Hernández, M.; Rico-Amorós, A.M.; Olcina-Cantos, J.; Moltó-Mantero, E. Extending Natural Limits to Address Water Scarcity? The Role of Non-Conventional Water Fluxes in Climate Change Adaptation Capacity: A Review. Sustainability 2021, 13, 2473. [Google Scholar] [CrossRef]
- Dolan, F.; Lamontagne, J.; Link, R.; Hejazi, M.; Reed, P.; Edmonds, J. Evaluating the economic impact of water scarcity in a changing world. Nat. Commun. 2021, 12, 1915. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Xia, W.; Huang, D.; Cheng, M.; Zhang, H.; Cai, T.; Xiong, W.; Yang, Y.; Song, B.; Wang, W.; et al. Strategies for enhancing the perylene diimide photocatalytic degradation activity: Method, effect factor, and mechanism. Environ. Sci. Nano 2021, 8, 602–618. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Isgoren, M.; Gengec, E.; Veli, S.; Hassandoost, R.; Khataee, A. The used automobile catalytic converter as an efficient catalyst for removal of malathion through wet air oxidation process. Int. J. Hydrog. Energy 2021, in press. [Google Scholar] [CrossRef]
- Hassandoost, R.; Pouran, S.R.; Khataee, A.; Orooji, Y.; Joo, S.W. Hierarchically structured ternary heterojunctions based on Ce3+/ Ce4+ modified Fe3O4 nanoparticles anchored onto graphene oxide sheets as magnetic visible-light-active photocatalysts for decontamination of oxytetracycline. J. Hazard. Mater. 2019, 376, 200–211. [Google Scholar] [CrossRef]
- Doustkhah, E.; Esmat, M.; Fukata, N.; Ide, Y.; Hanaor, D.A.H.; Assadi, M.H.N. MOF-derived nanocrystalline ZnO with controlled orientation and photocatalytic activity. Chemosphere 2022, 303, 134932. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Gao, H.; Liu, G.; Pu, Z.; Wang, S.; Yi, Z.; Wu, X.; Yang, H. Preparation of core-shell heterojunction photocatalysts by coating CdS nanoparticles onto Bi4Ti3O12 hierarchical microspheres and their photocatalytic removal of organic pollutants and Cr(VI) ions. Colloids Surf. Physicochem. Eng. Asp. 2022, 633, 127918. [Google Scholar] [CrossRef]
- Xiao, L.; Youji, L.; Feitai, C.; Peng, X.; Ming, L. Facile synthesis of mesoporous titanium dioxide doped by Ag-coated graphene with enhanced visible-light photocatalytic performance for methylene blue degradation. RSC Adv. 2017, 7, 25314–25324. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Gao, H.; Liu, G.; Wang, S.; Yi, Z.; Wu, X.; Yang, H. Synthesis of carnation flower-like Bi2O2CO3 photocatalyst and its promising application for photoreduction of Cr(VI). Adv. Powder Technol. 2022, 33, 103481. [Google Scholar] [CrossRef]
- Chen, P.; Liu, F.; Ding, H.; Chen, S.; Chen, L.; Li, Y.-J.; Au, C.-T.; Yin, S.-F. Porous double-shell CdS@C3N4 octahedron derived by in situ supramolecular self-assembly for enhanced photocatalytic activity. Appl. Catal. B Environ. 2019, 252, 33–40. [Google Scholar] [CrossRef]
- Ansarian, Z.; Khataee, A.; Arefi-Oskoui, S.; Orooji, Y.; Lin, H. Ultrasound-assisted catalytic activation of peroxydisulfate on Ti3GeC2 MAX phase for efficient removal of hazardous pollutants. Mater. Today Chem. 2022, 24, 100818. [Google Scholar] [CrossRef]
- Yousef Tizhoosh, N.; Khataee, A.; Hassandoost, R.; Darvishi Cheshmeh Soltani, R.; Doustkhah, E. Ultrasound-engineered synthesis of WS2@CeO2 heterostructure for sonocatalytic degradation of tylosin. Ultrason. Sonochem. 2020, 67, 105114. [Google Scholar] [CrossRef]
- Khataee, A.; Hassandoost, R.; Rahim Pouran, S. Cerium-substituted magnetite: Fabrication, characterization and sonocatalytic activity assessment. Ultrason. Sonochem. 2018, 41, 626–640. [Google Scholar] [CrossRef]
- Ahmadi, A.; Zarei, M.; Hassani, A.; Ebratkhahan, M.; Olad, A. Facile synthesis of iron(II) doped carbonaceous aerogel as a three-dimensional cathode and its excellent performance in electro-Fenton degradation of ceftazidime from water solution. Sep. Purif. Technol. 2021, 278, 119559. [Google Scholar] [CrossRef]
- Ebratkhahan, M.; Zarei, M.; Babaei, T.; Hosseini, M.G.; Hosseini, M.M.; Fathipour, Z. Efficient electrochemical removal of 5-fluorouracil pharmaceutical from wastewater by mixed metal oxides via anodic oxidation process. Chemosphere 2022, 296, 134007. [Google Scholar] [CrossRef]
- Wang, W.; Chen, M.; Wang, D.; Yan, M.; Liu, Z. Different activation methods in sulfate radical-based oxidation for organic pollutants degradation: Catalytic mechanism and toxicity assessment of degradation intermediates. Sci. Total Environ. 2021, 772, 145522. [Google Scholar] [CrossRef]
- Yu, F.; Poole III, D.; Mathew, S.; Yan, N.; Hessels, J.; Orth, N.; Ivanović-Burmazović, I.; Reek, J.N.H. Control over Electrochemical Water Oxidation Catalysis by Preorganization of Molecular Ruthenium Catalysts in Self-Assembled Nanospheres. Angew. Chem. Int. Ed. 2018, 57, 11247–11251. [Google Scholar] [CrossRef]
- Cao, W.; Lin, L.; Qi, H.; He, Q.; Wu, Z.; Wang, A.; Luo, W.; Zhang, T. In-situ synthesis of single-atom Ir by utilizing metal-organic frameworks: An acid-resistant catalyst for hydrogenation of levulinic acid to γ-valerolactone. J. Catal. 2019, 373, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Ding, Y.; Cao, X.; Tian, T.; Lin, J. Homogeneous and heterogeneous photocatalytic water oxidation by polyoxometalates containing the most earth-abundant transition metal, iron. Appl. Catal. B Environ. 2018, 237, 1091–1100. [Google Scholar] [CrossRef]
- Gheisari, H.; Karamian, E.; Abdellahi, M. A novel hydroxyapatite –Hardystonite nanocomposite ceramic. Ceram. Int. 2015, 41, 5967–5975. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, N. Removal of carbamazepine from aqueous solution using sono-activated persulfate process. Ultrason. Sonochem. 2016, 29, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Kolthoff, I.M.; Miller, I.K. The Chemistry of Persulfate. I. The Kinetics and Mechanism of the Decomposition of the Persulfate Ion in Aqueous Medium1. J. Am. Chem. Soc. 1951, 73, 3055–3059. [Google Scholar] [CrossRef]
- Wang, C.-W.; Liang, C. Oxidative degradation of TMAH solution with UV persulfate activation. Chem. Eng. J. 2014, 254, 472–478. [Google Scholar] [CrossRef]
- Hassandoost, R.; Kotb, A.; Movafagh, Z.; Esmat, M.; Guegan, R.; Endo, S.; Jevasuwan, W.; Fukata, N.; Sugahara, Y.; Khataee, A.; et al. Nanoarchitecturing bimetallic manganese cobaltite spinels for sonocatalytic degradation of oxytetracycline. Chem. Eng. J. 2022, 431, 133851. [Google Scholar] [CrossRef]
- Sisi, A.J.; Khataee, A.; Fathinia, M.; Vahid, B. Ultrasonic-assisted degradation of a triarylmethane dye using combined peroxydisulfate and MOF-2 catalyst: Synergistic effect and role of oxidative species. J. Mol. Liq. 2020, 297, 111838. [Google Scholar] [CrossRef]
- Hasija, V.; Raizada, P.; Sudhaik, A.; Sharma, K.; Kumar, A.; Singh, P.; Jonnalagadda, S.B.; Thakur, V.K. Recent advances in noble metal free doped graphitic carbon nitride based nanohybrids for photocatalysis of organic contaminants in water: A review. Appl. Mater. Today 2019, 15, 494–524. [Google Scholar] [CrossRef]
- Keerthana, S.P.; Yuvakkumar, R.; Kumar, P.S.; Ravi, G.; Velauthapillai, D. Rare earth metal (Sm) doped zinc ferrite (ZnFe2O4) for improved photocatalytic elimination of toxic dye from aquatic system. Environ. Res. 2021, 197, 111047. [Google Scholar] [CrossRef]
- Mohammadi, S.; Esmailpour, A.; Doustkhah, E.; Assadi, M.H.N. Stability Trends in Mono-Metallic 3d Layered Double Hydroxides. Nanomaterials 2022, 12, 1339. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, X.; Zhu, Y.; Zhang, Y.; Huang, C.-H. Synergistic activation of peroxydisulfate with magnetite and copper ion at neutral condition. Water Res. 2020, 186, 116371. [Google Scholar] [CrossRef] [PubMed]
- Arfanis, M.K.; Athanasekou, C.P.; Sakellis, E.; Boukos, N.; Ioannidis, N.; Likodimos, V.; Sygellou, L.; Bouroushian, M.; Kontos, A.G.; Falaras, P. Photocatalytic properties of copper—Modified core-shell titania nanocomposites. J. Photochem. Photobiol. A Chem. 2019, 370, 145–155. [Google Scholar] [CrossRef]
- Li, W.; Liu, B.; Wang, Z.; Wang, K.; Lan, Y.; Zhou, L. Efficient activation of peroxydisulfate (PDS) by rice straw biochar modified by copper oxide (RSBC-CuO) for the degradation of phenacetin (PNT). Chem. Eng. J. 2020, 395, 125094. [Google Scholar] [CrossRef]
- Feng, S.; Xiao, B.; Wu, M.; Wang, Y.; Chen, R.; Liu, H. Copper phosphide: A dual-catalysis-center catalyst for the efficient activation of peroxydisulfate and degradation of Orange II. Sep. Purif. Technol. 2020, 248, 117004. [Google Scholar] [CrossRef]





| Parameter | Range |
|---|---|
| Cu-THAp amount (g/L) | 0.25–1.25 |
| RhB Initail concentration (mg/L) | 5–60 |
| pH | 3–9 |
| PDS concentration (mmol/L) | 2.5–10 |
| Run Number | Cu Concentration (mg/L) |
|---|---|
| Blank sample | 0.01 |
| 1 | 0.58 |
| 2 | 0.53 |
| 3 | 0.47 |
| 4 | 0.49 |
| 5 | 0.41 |
| 6 | 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmani, F.; Ghadi, A.; Doustkhah, E.; Khaksar, S. In Situ Formation of Copper Phosphate on Hydroxyapatite for Wastewater Treatment. Nanomaterials 2022, 12, 2650. https://doi.org/10.3390/nano12152650
Rahmani F, Ghadi A, Doustkhah E, Khaksar S. In Situ Formation of Copper Phosphate on Hydroxyapatite for Wastewater Treatment. Nanomaterials. 2022; 12(15):2650. https://doi.org/10.3390/nano12152650
Chicago/Turabian StyleRahmani, Fatemeh, Arezoo Ghadi, Esmail Doustkhah, and Samad Khaksar. 2022. "In Situ Formation of Copper Phosphate on Hydroxyapatite for Wastewater Treatment" Nanomaterials 12, no. 15: 2650. https://doi.org/10.3390/nano12152650