Easy Diameter Tuning of Silicon Nanowires with Low-Cost SnO2-Catalyzed Growth for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Growing SiNWs from SnO2 Catalysts
3.2. Tuning the SiNW Diameter with Sn/Si Loading
3.3. Tuning SiNW Diameter with Inert Gas Additions
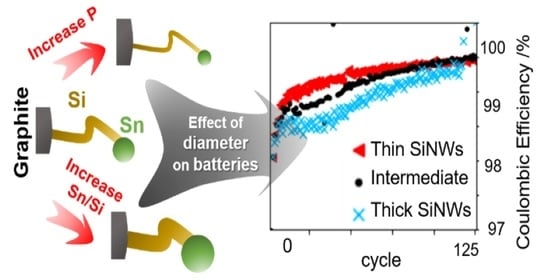
3.4. Electrochemistry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-Ion Battery Materials: Present and Future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Choi, J.W.; Aurbach, D. Promise and Reality of Post-Lithium-Ion Batteries with High Energy Densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Li, H.; Huang, X.; Chen, L.; Wu, Z.; Liang, Y. A High Capacity Nano—Si Composite Anode Material for Lithium Rechargeable Batteries. Electrochem. Solid-State Lett. 1999, 2, 547. [Google Scholar] [CrossRef]
- Graetz, J.; Ahn, C.C.; Yazami, R.; Fultz, B. Highly Reversible Lithium Storage in Nanostructured Silicon. Electrochem. Solid-State Lett. 2003, 6, A194. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhong, L.; Huang, S.; Mao, S.X.; Zhu, T.; Huang, J.Y. Size-Dependent Fracture of Silicon Nanoparticles During Lithiation. ACS Nano 2012, 6, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-Performance Lithium Battery Anodes Using Silicon Nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Yao, F.; Zamfir, M.R.; Biswas, C.; So, K.P.; Lee, Y.H.; Kim, S.M.; Cha, S.N.; Kim, J.M.; Pribat, D. Highly Interconnected Si Nanowires for Improved Stability Li-Ion Battery Anodes. Adv. Energy Mater. 2011, 1, 1154–1161. [Google Scholar] [CrossRef]
- Ryu, I.; Choi, J.W.; Cui, Y.; Nix, W.D. Size-Dependent Fracture of Si Nanowire Battery Anodes. J. Mech. Phys. Solids 2011, 59, 1717–1730. [Google Scholar] [CrossRef]
- Ma, Z.; Li, T.; Huang, Y.L.; Liu, J.; Zhou, Y.; Xue, D. Critical Silicon-Anode Size for Averting Lithiation-Induced Mechanical Failure of Lithium-Ion Batteries. RSC Adv. 2013, 3, 7398–7402. [Google Scholar] [CrossRef]
- Sun, F.; Tan, Z.; Hu, Z.; Chen, J.; Luo, J.; Wu, X.; Cheng, G.; Zheng, R. Ultrathin Silicon Nanowires Produced by a Bi-Metal-Assisted Chemical Etching Method for Highly Stable Lithium-Ion Battery Anodes. Nano 2020, 15, 2050076. [Google Scholar] [CrossRef]
- Keller, C.; Desrues, A.; Karuppiah, S.; Martin, E.; Alper, J.P.; Boismain, F.; Villevieille, C.; Herlin-Boime, N.; Haon, C.; Chenevier, P. Effect of Size and Shape on Electrochemical Performance of Nano-Silicon-Based Lithium Battery. Nanomaterials 2021, 11, 307. [Google Scholar] [CrossRef]
- Kasavajjula, U.; Wang, C.; Appleby, A.J. Nano- and Bulk-Silicon-Based Insertion Anodes for Lithium-Ion Secondary Cells. J. Power Sources 2007, 163, 1003–1039. [Google Scholar] [CrossRef]
- Yim, C.-H.; Niketic, S.; Salem, N.; Naboka, O.; Abu-Lebdeh, Y. Towards Improving the Practical Energy Density of Li-Ion Batteries: Optimization and Evaluation of Silicon:Graphite Composites in Full Cells. J. Electrochem. Soc. 2016, 164, A6294. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Gao, X.; Li, J.; Yuan, C. Life Cycle Environmental Impact of High-Capacity Lithium Ion Battery with Silicon Nanowires Anode for Electric Vehicles. Environ. Sci. Technol. 2014, 48, 3047–3055. [Google Scholar] [CrossRef]
- Wu, Z.; Kong, D. Comparative Life Cycle Assessment of Lithium-Ion Batteries with Lithium Metal, Silicon Nanowire, and Graphite Anodes. Clean Techn Environ. Policy 2018, 20, 1233–1244. [Google Scholar] [CrossRef]
- Karuppiah, S.; Keller, C.; Kumar, P.; Jouneau, P.-H.; Aldakov, D.; Ducros, J.-B.; Lapertot, G.; Chenevier, P.; Haon, C. A Scalable Silicon Nanowires-Grown-On-Graphite Composite for High-Energy Lithium Batteries. ACS Nano 2020, 14, 12006–12015. [Google Scholar] [CrossRef]
- Chenevier, P.; Reiss, P.; Burchak, O. Method for Producing Silicon Nanowires. EU Patent EP3154903A1, 12 June 2015. [Google Scholar]
- Burchak, O.; Keller, C.; Lapertot, G.; Salaün, M.; Danet, J.; Chen, Y.; Bendiab, N.; Pépin-Donat, B.; Lombard, C.; Faure-Vincent, J.; et al. Scalable Chemical Synthesis of Doped Silicon Nanowires for Energy Applications. Nanoscale 2019, 11, 22504–22514. [Google Scholar] [CrossRef]
- Schmidt, V.; Wittemann, J.V.; Senz, S.; Gösele, U. Silicon Nanowires: A Review on Aspects of Their Growth and Their Electrical Properties. Adv. Mater. 2009, 21, 2681–2702. [Google Scholar] [CrossRef]
- Dhalluin, F.; Desré, P.J.; den Hertog, M.I.; Rouvière, J.-L.; Ferret, P.; Gentile, P.; Baron, T. Critical Condition for Growth of Silicon Nanowires. J. Appl. Phys. 2007, 102, 094906. [Google Scholar] [CrossRef]
- Chockla, A.M.; Klavetter, K.C.; Mullins, C.B.; Korgel, B.A. Tin-Seeded Silicon Nanowires for High Capacity Li-Ion Batteries. Chem. Mater. 2012, 24, 3738–3745. [Google Scholar] [CrossRef]
- Bogart, T.D.; Oka, D.; Lu, X.; Gu, M.; Wang, C.; Korgel, B.A. Lithium Ion Battery Peformance of Silicon Nanowires with Carbon Skin. ACS Nano 2014, 8, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Loveridge, M.J.; Malik, R.; Paul, S.; Manjunatha, K.N.; Gallanti, S.; Tan, C.; Lain, M.; Roberts, A.J.; Bhagat, R. Binder-Free Sn–Si Heterostructure Films for High Capacity Li-Ion Batteries. RSC Adv. 2018, 8, 16726–16737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.-B.; Tsai, C.-Y.; Chen, K.-T.; Tuan, H.-Y. Solution-Grown Phosphorus-Hyperdoped Silicon Nanowires/Carbon Nanotube Bilayer Fabric as a High-Performance Lithium-Ion Battery Anode. ACS Appl. Energy Mater. 2021, 4, 3160–3168. [Google Scholar] [CrossRef]
- Imtiaz, S.; Amiinu, I.S.; Storan, D.; Kapuria, N.; Geaney, H.; Kennedy, T.; Ryan, K.M. Dense Silicon Nanowire Networks Grown on a Stainless-Steel Fiber Cloth: A Flexible and Robust Anode for Lithium-Ion Batteries. Adv. Mater. 2021, 33, 2105917. [Google Scholar] [CrossRef] [PubMed]
- Gilman, H.; Miles, D. Communications—Disproportionation Reaction of Diphenylsilane in the Absence of Any Added Catalyst. J. Org. Chem. 1958, 23, 326–328. [Google Scholar] [CrossRef]
- Lee, D.C.; Hanrath, T.; Korgel, B.A. The Role of Precursor-Decomposition Kinetics in Silicon-Nanowire Synthesis in Organic Solvents. Angew. Chem. Int. 3573–3577. [CrossRef]
- Schmidt, V.; Wittemann, J.V.; Gösele, U. Growth, Thermodynamics, and Electrical Properties of Silicon Nanowires. Chem. Rev. 2010, 110, 361–388. [Google Scholar] [CrossRef] [Green Version]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 93rd ed.; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9781439880494. [Google Scholar]
- Olesinski, R.W.; Abbaschian, G.J. The Si−Sn (Silicon−Tin) System. Bull. Alloy Phase Diagr. 1984, 5, 273–276. [Google Scholar] [CrossRef]
- Al-Taay, H.F.; Mahdi, M.A.; Parlevliet, D.; Jennings, P. Controlling the Diameter of Silicon Nanowires Grown Using a Tin Catalyst. Mater. Sci. Semicond. Process. 2013, 16, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Aminu, I.S.; Geaney, H.; Imtiaz, S.; Adegoke, T.E.; Kapuria, N.; Collins, G.A.; Ryan, K.M. A Copper Silicide Nanofoam Current Collector for Directly Grown Si Nanowire Networks and Their Application as Lithium-Ion Anodes. Adv. Funct. Mater. 2020, 30, 2003278. [Google Scholar] [CrossRef]
- Westwater, J.; Gosain, D.P.; Tomiya, S.; Usui, S.; Ruda, H. Growth of Silicon Nanowires via Gold/Silane Vapor–Liquid–Solid Reaction. J. Vac. Sci. Technol. B Microelectron. Nanometer. Struct. Process. Meas. Phenom. 1998, 15, 554. [Google Scholar] [CrossRef]
- Reiss, P.; Carrière, M.; Lincheneau, C.; Vaure, L.; Tamang, S. Synthesis of Semiconductor Nanocrystals, Focusing on Nontoxic and Earth-Abundant Materials. Chem. Rev. 2016, 116, 10731–10819. [Google Scholar] [CrossRef]
- Yu, L.; Alet, P.-J.; Picardi, G.; Maurin, I.; Roca i Cabarrocas, P.R. Synthesis, morphology and compositional evolution of silicon nanowires directly grown on SnO2 substrates. Nanotechnology 2008, 19, 485605. [Google Scholar] [CrossRef]
- Xu, T.; Lambert, Y.; Krzeminski, C.; Grandidier, B.; Stiévenard, D.; Lévêque, G.; Akjouj, A.; Pennec, Y.; Djafari-Rouhani, B. Optical Absorption of Silicon Nanowires. J. Appl. Phys. 2012, 112, 033506. [Google Scholar] [CrossRef] [Green Version]
- Dhalluin, F.; Baron, T.; Ferret, P.; Salem, B.; Gentile, P.; Harmand, J.C. Silicon Nanowires: Diameter Dependence of Growth Rate and Delay in Growth. Appl. Phys. Lett. 2010, 96, 133109. [Google Scholar] [CrossRef]
- O’Neal, H.E.; Ring, M.A.; Kim, D.; King, K.D. Primary Reaction Channels and Kinetics of the Thermal Decomposition of Phenylsilane. J. Phys. Chem 1995, 99, 9397–9402. [Google Scholar] [CrossRef]
- Nájera, J.J.; Cáceres, J.O.; Lane, S.I. Gas-Phase Infrared Laser Photolysis of Phenylsilane. J. Photochem. Photobiol. A Chem. 2000, 131, 1–11. [Google Scholar] [CrossRef]
- Hamidinezhad, H. Thickness Effect of Catalyst Layer on Silicon Nanowires Morphology and Features. Appl. Surf. Sci. 2016, 364, 484–489. [Google Scholar] [CrossRef]
- Sharma, S.; Kamins, T.I.; Williams, R.S. Synthesis of Thin Silicon Nanowires Using Gold-Catalyzed Chemical Vapor Deposition. Appl. Phys. A 2005, 80, 1225–1229. [Google Scholar] [CrossRef]
- Gohier, A.; Laïk, B.; Pereira-Ramos, J.-P.; Cojocaru, C.S.; Tran-Van, P. Influence of the Diameter Distribution on the Rate Capability of Silicon Nanowires for Lithium-Ion Batteries. J. Power Sources 2012, 203, 135–139. [Google Scholar] [CrossRef] [Green Version]
- Langklotz, U.; Lein, T.; Schulze, C.; Weiser, M.; Krause, A.; Michaelis, A. Scalable Fabrication of Gold Nanoparticles with Adjustable Size Distribution as Catalytic Nuclei for the CVD Growth of Silicon Nanowires. Appl. Surf. Sci. 2020, 502, 144203. [Google Scholar] [CrossRef]
- Woodard, J.C.; Kalisvaart, W.P.; Sayed, S.Y.; Olsen, B.C.; Buriak, J.M. Beyond Thin Films: Clarifying the Impact of c-Li15Si4 Formation in Thin Film, Nanoparticle, and Porous Si Electrodes. ACS Appl. Mater. Interfaces 2021, 13, 38147–38160. [Google Scholar] [CrossRef]
- Schott, T.; Robert, R.; Pacheco Benito, S.; Ulmann, P.A.; Lanz, P.; Zürcher, S.; Spahr, M.E.; Novák, P.; Trabesinger, S. Cycling Behavior of Silicon-Containing Graphite Electrodes, Part B: Effect of the Silicon Source. J. Phys. Chem. C 2017, 121, 25718–25728. [Google Scholar] [CrossRef]
- Schulze, M.C.; Carroll, G.M.; Martin, T.R.; Sanchez-Rivera, K.; Urias, F.; Neale, N.R. Hydrophobic versus Hydrophilic Interfacial Coatings on Silicon Nanoparticles Teach Us How to Design the Solid Electrolyte Interphase in Silicon-Based Li-Ion Battery Anodes. ACS Appl. Energy Mater. 2021, 4, 1628–1636. [Google Scholar] [CrossRef]
- Shen, Q.; Zheng, R.; Lv, Y.; Lou, Y.; Shi, L.; Yuan, S. Scalable Fabrication of Silicon-Graphite Microsphere by Mechanical Processing for Lithium-Ion Battery Anode with Large Capacity and High Cycling Stability. Batter. Supercaps 2022, e202200186. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Zhang, J.; Zhao, Y.; Zhao, P.; Ni, L.; Xie, Q.; Meng, J. Ball-Milled Silicon with Amorphous Al2O3/C Hybrid Coating Embedded in Graphene/Graphite Nanosheets with a Boosted Lithium Storage Capability. Langmuir 2022, 38, 8555–8563. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.M.; Batmaz, R.; Li, J.; Wang, X.; Xiao, X.; Yu, A.; Chen, Z. Evidence of Covalent Synergy in Silicon–Sulfur–Graphene Yielding Highly Efficient and Long-Life Lithium-Ion Batteries. Nat. Commun. 2015, 6, 8597. [Google Scholar] [CrossRef]
- Kilian, S.; Adegoke, T.E.; Ahad, S.A.; Geaney, H.; Kennedy, T.; Ryan, K.M. Temperature induced diameter variation of silicon nanowires via a liquid–solid phase transition in the Zn seed. Chem. Commun. 2021, 57, 12504–12507. Available online: https://pubs.rsc.org/en/content/articlelanding/2021/CC/D1CC04427C (accessed on 27 June 2022). [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keller, C.; Djezzar, Y.; Wang, J.; Karuppiah, S.; Lapertot, G.; Haon, C.; Chenevier, P. Easy Diameter Tuning of Silicon Nanowires with Low-Cost SnO2-Catalyzed Growth for Lithium-Ion Batteries. Nanomaterials 2022, 12, 2601. https://doi.org/10.3390/nano12152601
Keller C, Djezzar Y, Wang J, Karuppiah S, Lapertot G, Haon C, Chenevier P. Easy Diameter Tuning of Silicon Nanowires with Low-Cost SnO2-Catalyzed Growth for Lithium-Ion Batteries. Nanomaterials. 2022; 12(15):2601. https://doi.org/10.3390/nano12152601
Chicago/Turabian StyleKeller, Caroline, Yassine Djezzar, Jingxian Wang, Saravanan Karuppiah, Gérard Lapertot, Cédric Haon, and Pascale Chenevier. 2022. "Easy Diameter Tuning of Silicon Nanowires with Low-Cost SnO2-Catalyzed Growth for Lithium-Ion Batteries" Nanomaterials 12, no. 15: 2601. https://doi.org/10.3390/nano12152601