β-Ga2O3 Nanostructures: Chemical Vapor Deposition Growth Using Thermally Dewetted Au Nanoparticles as Catalyst and Characterization
Abstract
:1. Introduction
2. Experimental Methods
3. Results and Discussion
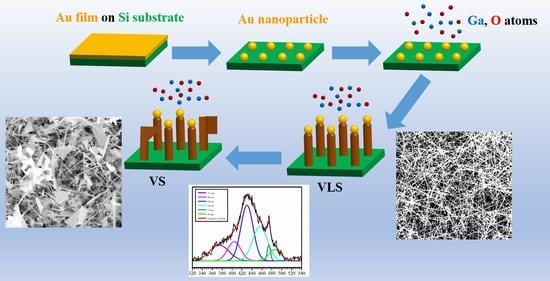
3.1. Morphological and Structural Characterization and Growth Mechanism
3.2. Bandgap Analysis
3.3. Photoluminescence Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamaga, M.; Villora, E.G.; Shimamura, K.; Ichinose, N.; Honda, M. Donor Structure and Electric Transport Mechanism in β-Ga2O3. Phys. Rev. B 2003, 68, 155207. [Google Scholar] [CrossRef]
- Kumar, S.; Tessarek, C.; Sarau, G.; Christiansen, S.; Singh, R. Self-Catalytic Growth of β-Ga2O3 Nanostructures by Chemical Vapor Deposition. Adv. Eng. Mater. 2015, 17, 709–715. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R. Nanofunctional Gallium Oxide (Ga2O3) Nanowires/Nanostructures and Their Applications in Nanodevices. Phys. Status Solidi-Rapid Res. Lett. 2013, 7, 781–792. [Google Scholar] [CrossRef]
- Li, Y.B.; Tokizono, T.; Liao, M.Y.; Zhong, M.A.; Koide, Y.; Yamada, I.; Delaunay, J.J. Efficient Assembly of Bridged β-Ga2O3 Nanowires for Solar-Blind Photodetection. Adv. Funct. Mater. 2010, 20, 3972–3978. [Google Scholar] [CrossRef]
- Li, L.; Auer, E.; Liao, M.Y.; Fang, X.S.; Zhai, T.Y.; Gautam, U.K.; Lugstein, A.; Koide, Y.; Bando, Y.; Golberg, D. Deep-ultraviolet Solar-blind Photoconductivity of Individual Gallium Oxide Nanobelts. Nanoscale 2011, 3, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.P.; Prokes, S.M.; Perkins, F.K.; Zaghloul, M.E. Design and Performance of a Simple, Room-temperature Ga2O3 Nanowire Gas Sensor. Appl. Phys. Lett. 2009, 95, 103102. [Google Scholar] [CrossRef]
- Mazeina, L.; Bermudez, V.M.; Perkins, F.K.; Arnold, S.P.; Prokes, S.M. Interaction of Functionalized Ga2O3 NW-based Room Temperature Gas Sensors with Different Hydrocarbons. Sens. Actuators B 2010, 151, 114–120. [Google Scholar] [CrossRef]
- Chang, P.C.; Fan, Z.Y.; Tseng, W.Y.; Rajagopal, A.; Lu, J.G. β-Ga2O3 Nanowires: Synthesis, Characterization, and p-channel Field-effect Transistor. Appl. Phys. Lett. 2005, 87, 2221002. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Chou, L.J.; Lin, G.R.; Bando, Y.; Golberg, D. Nanophotonic Switch: Gold-in-Ga2O3 Peapod Nanowires. Nano Lett. 2008, 8, 3081–3085. [Google Scholar] [CrossRef]
- Nogales, E.; Garcia, J.A.; Mendez, B.; Piqueras, J. Doped Gallium Oxide Nanowires with Waveguiding Behavior. Appl. Phys. Lett. 2007, 91, 133108. [Google Scholar] [CrossRef] [Green Version]
- Vanithakumari, S.C.; Nanda, K.K. A One-Step Method for the Growth of Ga2O3-Nanorod-Based White-Light-Emitting Phosphors. Adv. Mater. 2009, 21, 3581–3584. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Lan, C.Y.; Wei, R.J.; Ho, J.C. Transparent Metal-oxide Nanowires and Their Applications in Harsh Electronics. J. Mater. Chem. C 2019, 7, 202–217. [Google Scholar] [CrossRef]
- Binet, L.; Gourier, D. Origin of the Blue Luminescence of β-Ga2O3. J. Phys. Chem. Solids 1998, 59, 1241–1249. [Google Scholar] [CrossRef]
- Villora, E.G.; Atou, T.; Sekiguchi, T.; Sugawara, T.; Kikuchi, M.; Fukuda, T. Cathodoluminescence of Undoped β-Ga2O3 Single Crystals. Solid State Commun. 2001, 120, 455–458. [Google Scholar] [CrossRef]
- Harwig, T.; Kellendonk, F.; Slappendel, S. Ultraviolet Luminescence of β-Gallium Sesquioxide. J. Phys. Chem. Solids 1978, 39, 675–680. [Google Scholar] [CrossRef]
- Dong, L.P.; Jia, R.X.; Xin, B.; Peng, B.; Zhang, Y.M. Effects of Oxygen Vacancies on the Structural and Optical Properties of β-Ga2O3. Sci. Rep. 2017, 7, 40160. [Google Scholar] [CrossRef]
- Wang, Y.S.; Dickens, P.T.; Varley, J.B.; Ni, X.J.; Lotubai, E.; Sprawls, S.; Liu, F.; Lordi, V.; Krishnamoorthy, S.; Blair, S.; et al. Incident Wavelength and Polarization Dependence of Spectral Shifts in β-Ga2O3 UV Photoluminescence. Sci. Rep. 2018, 8, 18075. [Google Scholar] [CrossRef] [Green Version]
- Harwig, T.; Kellendonk, F. Some Observations on Photoluminescence of Doped β-Gallium Sesquioxide. J. Solid State Chem. 1978, 24, 255–263. [Google Scholar] [CrossRef]
- Wu, X.C.; Song, W.H.; Huang, W.D.; Pu, M.H.; Zhao, B.; Sun, Y.P.; Du, J.J. Crystalline Gallium Oxide Nanowires: Intensive Blue Light Emitters. Chem. Phys. Lett. 2000, 328, 5–9. [Google Scholar] [CrossRef]
- Liang, C.H.; Meng, G.W.; Wang, G.Z.; Wang, Y.W.; Zhang, L.D.; Zhang, S.Y. Catalytic Synthesis and Photoluminescence of β-Ga2O3 Nanowires. Appl. Phys. Lett. 2001, 78, 3202–3204. [Google Scholar] [CrossRef]
- Dai, L.; Chen, X.L.; Zhang, X.N.; Jin, A.Z.; Zhou, T.; Hu, B.Q.; Zhang, Z. Growth and Optical Characterization of Ga2O3 Nanobelts and Nanosheets. J. Appl. Phys. 2002, 92, 1062–1064. [Google Scholar] [CrossRef]
- Ho, C.H.; Tseng, C.Y.; Tien, L.C. Thermoreflectance Characterization of β-Ga2O3 Thin-film Nanostrips. Opt. Express 2010, 18, 16360–16369. [Google Scholar] [CrossRef]
- Terasako, T.; Kawasaki, Y.; Yagi, M. Growth and Morphology Control of β-Ga2O3 Nanostructures by Atmospheric-pressure CVD. Thin Solid Film. 2016, 620, 23–29. [Google Scholar] [CrossRef]
- Song, Y.P.; Zhang, H.Z.; Lin, C.; Zhu, Y.W.; Li, G.H.; Yang, F.H.; Yu, D.P. Luminescence Emission Originating from Nitrogen Doping of β-Ga2O3 Nanowires. Phys. Rev. B 2004, 69, 075304. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Kong, Y.C.; Wang, Y.Z.; Du, X.; Bai, Z.G.; Wang, J.J.; Yu, D.P.; Ding, Y.; Hang, Q.L.; Feng, S.Q. Ga2O3 Nanowires Prepared by Physical Evaporation. Solid State Commun. 1999, 109, 677–682. [Google Scholar] [CrossRef]
- Gao, Y.H.; Bando, Y.; Sato, T.; Zhang, Y.F.; Gao, X.Q. Synthesis, Raman Scattering and Defects of β-Ga2O3 Nanorods. Appl. Phys. Lett. 2002, 81, 2267–2269. [Google Scholar] [CrossRef]
- Choi, Y.C.; Kim, W.S.; Park, Y.S.; Lee, S.M.; Bae, D.J.; Lee, Y.H.; Park, G.S.; Choi, W.B.; Lee, N.S.; Kim, J.M. Catalytic Growth of β-Ga2O3 Nanowires by Arc Discharge. Adv. Mater. 2000, 12, 746–750. [Google Scholar] [CrossRef]
- Han, W.Q.; Kohler-Redlich, P.; Ernst, F.; Ruhle, M. Growth and Microstructure of Ga2O3 Nanorods. Solid State Commun. 2000, 115, 527–529. [Google Scholar] [CrossRef]
- Hu, J.Q.; Li, Q.; Meng, X.M.; Lee, C.S.; Lee, S.T. Synthesis of β-Ga2O3 Nanowires by Laser Ablation. J. Phys. Chem. B 2002, 106, 9536–9539. [Google Scholar] [CrossRef]
- Sharma, S.; Sunkara, M.K. Direct Synthesis of Gallium Oxide Tubes, Nanowires, and Nanopaintbrushes. J. Am. Chem. Soc. 2002, 124, 12288–12293. [Google Scholar] [CrossRef]
- Zhu, F.; Yang, Z.X.; Zhou, W.M.; Zhang, Y.F. Synthesis of β-Ga2O3 Nanowires Through Microwave Plasma Chemical Vapor Deposition. Appl. Surf. Sci. 2006, 252, 7930–7933. [Google Scholar] [CrossRef]
- Auer, E.; Lugstein, A.; Loffler, S.; Hyun, Y.J.; Brezna, W.; Bertagnolli, E.; Pongratz, P. Ultrafast VLS Growth of Epitaxial β-Ga2O3 Nanowires. Nanotechnology 2009, 20, 434017. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.C.; Aloni, S.; McCready, D.E.; Bourret-Courchesne, E.D. Controlled Vapor-liquid-solid Growth of Indium, Gallium, and Tin Oxide Nanowires via Chemical Vapor Transport. Cryst. Growth Des. 2006, 6, 1936–1941. [Google Scholar] [CrossRef]
- Hosein, I.D.; Hegde, M.; Jones, P.D.; Chirmanov, V.; Radovanovic, P.V. Evolution of the Faceting, Morphology and Aspect Ratio of Gallium Oxide Nanowires Grown by Vapor-solid Deposition. J. Cryst. Growth 2014, 396, 24–32. [Google Scholar] [CrossRef]
- Du, J.Y.; Xing, J.; Ge, C.; Liu, H.; Liu, P.Y.; Hao, H.Y.; Dong, J.J.; Zheng, Z.Y.; Gao, H. Highly Sensitive and Ultrafast Deep UV Photodetector Based on a β-Ga2O3 Nanowire Network Grown by CVD. J. Phys. D 2016, 49, 425105. [Google Scholar] [CrossRef]
- Bonvicini, S.N.; Fu, B.; Fulton, A.J.; Jia, Z.T.; Shi, Y.J. Formation of Au, Pt, and bimeallic Au-Pt Nanostructures From Thermal Dewetting of Single-layer or Bilayer Thin Films. Nanotechnology 2022, 33, 235604. [Google Scholar] [CrossRef]
- Ly, L.Q.; Fulton, A.J.; Bonvicini, S.N.; Shi, Y.J. Pulsed Laser-induced Dewetting and Thermal Dewetting of Ag Thin Films for the Fabrication of Ag Nanoparticles. Nanotechnology 2021, 32, 335301. [Google Scholar] [CrossRef]
- El-Sayed, H.A.; Horwood, C.A.; Owusu-Ansah, E.; Shi, Y.J.; Birss, V.I. Gold Nanoparticle Array Formation on Dimpled Ta Templates Using Pulsed Laser-induced Thin Film Dewetting. Phys. Chem. Chem. Phys. 2015, 17, 11062–11069. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, V.; Singh, T.; Hahnel, A.; Singh, R. The Effect of Deposition Time on the Structural and Optical Properties of β-Ga2O3 Nanowires Grown using CVD Technique. J. Nanopart. Res. 2014, 16, 2189. [Google Scholar] [CrossRef]
- Dohy, D.; Lucazeau, G.; Revcolevschi, A. Raman Spectra and Valence Force Field of Single Crystalline β-Ga2O3. J. Solid State Chem. 1982, 45, 180–192. [Google Scholar] [CrossRef]
- Rao, R.; Rao, A.M.; Xu, B.; Dong, J.; Sharma, S.; Sunkara, M.K. Blueshifted Raman Scattering and its Correlation with the [110] Growth Direction in Gallium Oxide Nanowires. J. Appl. Phys. 2005, 98, 094312. [Google Scholar] [CrossRef]
- Kumar, S.; Sarau, G.; Tessarek, C.; Bashouti, M.Y.; Hahnel, A.; Christiansen, S.; Singh, R. Study of Iron-catalysed Growth of β-Ga2O3 Nanowires and Their Detailed Characterization Using TEM, Raman and Cathodoluminescence Techniques. J. Phys. D 2014, 47, 435101. [Google Scholar] [CrossRef]
- Zhu, F.; Yang, Z.X.; Zhou, W.M.; Zhang, Y.F. Large Well-aligned Nanostructures of β-Ga2O3 Synthesized by Microwave Plasma Chemical Vapor Deposition. Appl. Surf. Sci. 2006, 253, 1655–1659. [Google Scholar] [CrossRef]
- Dai, Z.R.; Pan, Z.W.; Wang, Z.L. Gallium Oxide Nanoribbons and Nanosheets. J. Phys. Chem. B 2002, 106, 902–904. [Google Scholar] [CrossRef] [Green Version]
- Makula, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Kumar, V.; Singh, R. Diameter Tuning of β-Ga2O3 Nanowires Using Chemical Vapor Deposition Technique. Nanoscale Res. Lett. 2017, 12, 184. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.X.; Park, J.; Kong, X.Y.; Wilson, P.R.; Chen, Z.X.; Ahn, J.H. Facile Synthesis and Characterization of Gallium Oxide (β-Ga2O3) 1D Nanostructures: Nanowires, Nanoribbons, and Nanosheets. Cryst. Growth Des. 2008, 8, 1940–1944. [Google Scholar] [CrossRef]
- Nogales, E.; Mendez, B.; Piqueras, J. Cathodoluminescence from β-Ga2O3 Nanowires. Appl. Phys. Lett. 2005, 86, 113112. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.W.; Wu, J.J. Low-temperature Growth Well-aligned β-Ga2O3 Nanowires from a Single-source Organometallic Precursor. Adv. Mater. 2004, 16, 545–549. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, A.; Fu, B.; Bonvicini, S.N.; Ly, L.Q.; Jia, Z.; Shi, Y. β-Ga2O3 Nanostructures: Chemical Vapor Deposition Growth Using Thermally Dewetted Au Nanoparticles as Catalyst and Characterization. Nanomaterials 2022, 12, 2589. https://doi.org/10.3390/nano12152589
Yadav A, Fu B, Bonvicini SN, Ly LQ, Jia Z, Shi Y. β-Ga2O3 Nanostructures: Chemical Vapor Deposition Growth Using Thermally Dewetted Au Nanoparticles as Catalyst and Characterization. Nanomaterials. 2022; 12(15):2589. https://doi.org/10.3390/nano12152589
Chicago/Turabian StyleYadav, Asha, Bo Fu, Stephanie Nicole Bonvicini, Linh Quy Ly, Zhitai Jia, and Yujun Shi. 2022. "β-Ga2O3 Nanostructures: Chemical Vapor Deposition Growth Using Thermally Dewetted Au Nanoparticles as Catalyst and Characterization" Nanomaterials 12, no. 15: 2589. https://doi.org/10.3390/nano12152589