The Synthesis of Manganese Hydroxide Nanowire Arrays for a High-Performance Zinc-Ion Battery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SDS-Mn(OH)2 on CC
2.3. Structural Characterization
2.4. Fabrication of CC@Mn(OH)2//Zn Coin Cells
2.5. Electrochemical Tests
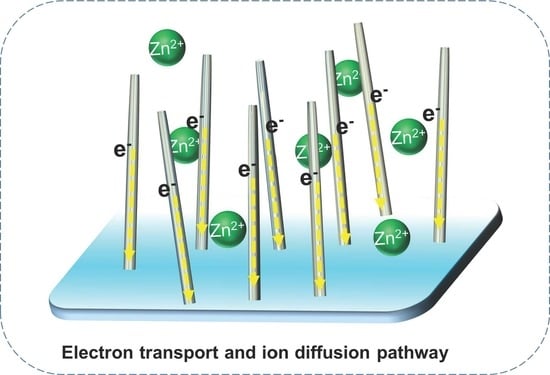
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Stor. Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Zubi, G.; Dufo-Lopez, R.; Carvalho, M.; Pasaoglu, G. The lithium-ion battery: State of the art and future perspectives. Renew. Sust. Energ. Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From Lithium-Ion to Sodium-Ion Batteries: Advantages, Challenges, and Surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Shan, L.; Liang, S.; Zhou, J. Issues and opportunities facing aqueous zinc-ion batteries. Energy Environ. Sci. 2019, 12, 3288–3304. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Z.; Hou, M.; Dong, X.; Liu, Y.; Wang, Y.; Xia, Y. Polyaniline-intercalated manganese dioxide nanolayers as a high-performance cathode material for an aqueous zinc-ion battery. Nat. Commun. 2018, 9, 2906. [Google Scholar] [CrossRef]
- Fang, G.; Zhou, J.; Pan, A.; Liang, S. Recent Advances in Aqueous Zinc-Ion Batteries. ACS Energy Lett. 2018, 3, 2480–2501. [Google Scholar] [CrossRef]
- Vo Pham Hoang, H.; Yong Nam, A.; Hur, J. Recent Advances in Transition Metal Dichalcogenide Cathode Materials for Aqueous Rechargeable Multivalent Metal-Ion Batteries. Nanomaterials 2021, 11, 1517. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Lu, Q.; Hantusch, M.; Zhang, H.; Qu, Z.; Tang, H.; Dong, H.; Schmidt, O.G.; Holze, R.; et al. Highly enhanced reversibility of a Zn anode by in-situ texturing. Energy Stor. Mater. 2022, 47, 98–104. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, C.; Du, Y.; Wang, X.; Ding, L.; Omar, A.; Mikhailova, D. Uniform Zn Deposition Achieved by Ag Coating for Improved Aqueous Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 16869–16875. [Google Scholar] [CrossRef]
- Cao, X.; Xia, H.; Zhao, X. Toward dendrite-free alkaline zinc-based rechargeable batteries: A minireview. Funct. Mater. Lett. 2019, 12, 1930004. [Google Scholar] [CrossRef]
- Gao, X.; Li, H.; Cao, X.; Lu, X. Mn3O4@MnS composite nanoparticles as cathode materials for aqueous rechargeable Zn ion batteries. Funct. Mater. Lett. 2021, 14, 2143002. [Google Scholar] [CrossRef]
- Qiu, W.; Li, Y.; You, A.; Zhang, Z.; Li, G.; Lu, X.; Tong, Y. High-performance flexible quasi-solid-state Zn-MnO2 battery based on MnO2 nanorod arrays coated 3D porous nitrogen-doped carbon cloth. J. Mater. Chem. A 2017, 5, 14838–14846. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, G.; Yan, M.; Xiong, T.; He, P.; He, L.; Xu, X.; Mai, L. Graphene Scroll-Coated alpha-MnO2 Nanowires as High-Performance Cathode Materials for Aqueous Zn-Ion Battery. Small 2018, 14, 1703850. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.; Zhou, W.; Ye, C.; Zhang, Q.; Chen, Y.; Gu, L.; Davey, K.; Qiao, S.-Z. An Electrolytic Zn-MnO2 Battery for High-Voltage and Scalable Energy Storage. Angew. Chem. Int. Ed. 2019, 58, 7823–7828. [Google Scholar] [CrossRef] [PubMed]
- Lian, S.; Sun, C.; Xu, W.; Huo, W.; Luo, Y.; Zhao, K.; Yao, G.; Xu, W.; Zhang, Y.; Li, Z.; et al. Built-in oriented electric field facilitating durable Zn-MnO2 battery. Nano Energy 2019, 62, 79–84. [Google Scholar] [CrossRef]
- Wu, Y.; Tao, Y.; Zhang, X.; Zhang, K.; Chen, S.; Liu, Y.; Ding, Y.; Cai, M.; Liu, X.; Dai, S. Self-assembled alpha-MnO2 urchin-like microspheres as a high-performance cathode for aqueous Zn-ion batteries. Sci. China Mater. 2020, 63, 1196–1204. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ye, F.; Wu, Z.; Jiang, L.; Zhang, L.; Hu, L. Macroporous, Freestanding Birnessite H0.08MnO2 center dot 0.7H2O Nanobelts/Carbon Nanotube Membranes for Wearable Zinc-Ion Batteries with Superior Rate Capability and Cyclability. ACS Appl. Energy Mater. 2021, 4, 4138–4149. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, Z.; Wu, X.; Wen, Y.; Chen, H.; Ni, X.; Liu, G.; Huang, J.; Peng, S. MnO2 cathode materials with the improved stability via nitrogen doping for aqueous zinc-ion batteries. J. Energy Chem. 2022, 64, 23–32. [Google Scholar] [CrossRef]
- Mao, F.; Li, Y.; Zou, Z.; Huang, B.; Yang, J.; Yao, J. Zn2+ storage performance and structural change of orthorhombic V2O5 nanowires as the cathode material for rechargeable aqueous zinc-ion batteries. Electrochim. Acta 2021, 397, 139255. [Google Scholar] [CrossRef]
- Li, J.; McColl, K.; Lu, X.; Sathasivam, S.; Dong, H.; Kang, L.; Li, Z.; Zhao, S.; Kafizas, A.G.; Wang, R.; et al. Multi-Scale Investigations of delta-Ni0.25V2O5 center dot nH2O Cathode Materials in Aqueous Zinc-Ion Batteries. Adv. Energy Mater. 2020, 10, 2000058. [Google Scholar] [CrossRef]
- Du, Y.-H.; Liu, X.-Y.; Wang, X.-Y.; Sun, J.-C.; Lu, Q.-Q.; Wang, J.-Z.; Omar, A.; Mikhailova, D. Freestanding strontium vanadate/carbon nanotube films for long-life aqueous zinc-ion batteries. Rare Metals 2022, 41, 415–424. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, X.; Lei, S.; Dai, X.; Xu, X.; Shi, W.; Liu, W.; Wu, F.; Cao, X. MXene for aqueous zinc-based energy storage devices. Funct. Mater. Lett. 2021, 14, 2130011. [Google Scholar] [CrossRef]
- Sha, D.; Lu, C.; He, W.; Ding, J.; Zhang, H.; Bao, Z.; Cao, X.; Fan, J.; Dou, Y.; Pan, L.; et al. Surface Selenization Strategy for V2CTx MXene toward Superior Zn-Ion Storage. ACS Nano 2022, 16, 2711–2720. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Li, H.; Zhang, K.; Zhang, Z.; Cao, J.; Shao, Z.; Tang, C.; Fu, S.; Wang, Q.; Wu, X. Zinc-Ion Storage Mechanism of Polyaniline for Rechargeable Aqueous Zinc-Ion Batteries. Nanomaterials 2022, 12, 1438. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, X.; Meng, Y.; Yu, M.; Yi, J.; Wu, Y.; Lu, X.; Tong, Y. Achieving Ultrahigh Energy Density and Long Durability in a Flexible Rechargeable Quasi-Solid-State Zn-MnO2 Battery. Adv. Mater. 2017, 29, 1700274. [Google Scholar] [CrossRef]
- Xu, C.; Li, B.; Du, H.; Kang, F. Energetic Zinc Ion Chemistry: The Rechargeable Zinc Ion Battery. Angew. Chem. Int. Ed. 2012, 51, 933–935. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, J.; Bai, C.; Li, X.; Fang, G.; Liang, S. Zn/MnO2 battery chemistry with dissolution-deposition mechanism. Mater. Today Energy 2020, 16, 100396. [Google Scholar] [CrossRef]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, X.; Li, H.; Cui, C.; Fu, C.; Zeng, S.; Wang, L. A controlled synthesis of gamma-MnOOH nanorods via a facile hydrothermal method for high-performance Li-ion batteries. CrystEngComm 2021, 23, 2376–2383. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, C.; Zhang, K.; Li, H.; Cao, J.; Shao, Z.; Gong, J. Synthesis of Mn(OH)2 Nanosheets on Carbon Cloth for High-Performance Aqueous Zinc-Ion Battery. J. Nanoelectron. Optoe. 2021, 16, 1698–1704. [Google Scholar] [CrossRef]
- Yang, Z.; Gong, J.; Tang, C.; Zhu, W.; Cheng, Z.; Jiang, J.; Ma, A.; Ding, Q. Vertically-aligned Mn(OH)2 nanosheet films for flexible all-solid-state electrochemical supercapacitors. J. Mater. Sci. Mater. Electron. 2017, 28, 17533–17540. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X.; Liu, H.; Zhao, D.; Wu, X. NiCo layered double hydroxide nanosheets with enhanced electrochemical performance. J. Alloys Compd. 2022, 903, 163926. [Google Scholar] [CrossRef]
- Li, J.-C.; Gong, J.; Zhang, X.; Lu, L.; Liu, F.; Dai, Z.; Wang, Q.-J.; Hong, X.; Pang, H.; Han, M. Alternate Integration of Vertically Oriented CuSe@FeOOH and CuSe@MnOOH Hybrid Nanosheets Frameworks for Flexible In-Plane Asymmetric Micro-supercapacitors. ACS Appl. Energy Mater. 2020, 3, 3692–3703. [Google Scholar] [CrossRef]
- Gong, J.; Tian, Y.; Yang, Z.; Wang, Q.; Hong, X.; Ding, Q. High-Performance Flexible All-Solid-State Asymmetric Supercapacitors Based on Vertically Aligned CuSe@Co(OH)2 Nanosheet Arrays. J. Phys. Chem. C 2018, 122, 2002–2011. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Li, J.-C.; Yang, J.; Zhao, S.; Yang, Z.; Zhang, K.; Bao, J.; Pang, H.; Han, M. High-Performance Flexible In-Plane Micro-Supercapacitors Based on Vertically Aligned CuSe@Ni(OH)2 Hybrid Nanosheet Films. ACS Appl. Mater. Interfaces 2018, 10, 38341–38349. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Jin, X.; Han, Y.; Lin, Y.; Lei, Z.; Wang, S.; Qin, L.; Jiao, S.; Cao, R. A Hollow-Structured Manganese Oxide Cathode for Stable Zn-MnO2 Batteries. Nanomaterials 2018, 8, 301. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.-R.; Xie, C.-P.; Wen, Y.; Tang, A.-P.; Song, H.-S. Mn(OH)2 electrodeposited on secondary porous Ni nano-architecture foam as high-performance electrode for supercapacitors. Ionics 2019, 25, 3287–3298. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Banerjee, D. Interpretation of XPS Mn(2p) spectra of Mn oxyhydroxides and constraints on the mechanism of MnO2 precipitation. Am. Mineral. 1998, 83, 305–315. [Google Scholar] [CrossRef]
- Anandan, S.; Gnana Sundara Raj, B.; Lee, G.-J.; Wu, J.J. Sonochemical synthesis of manganese (II) hydroxide for supercapacitor applications. Mater. Res. Bull. 2013, 48, 3357–3361. [Google Scholar] [CrossRef]
- Jia, D.; Li, Q.; Hanna, K.; Mailhot, G.; Brigante, M. Efficient removal of estrogenic compounds in water by MnIII-activated peroxymonosulfate: Mechanisms and application in sewage treatment plant water. Environ. Pollut. 2021, 288, 117728. [Google Scholar] [CrossRef] [PubMed]
- Ilton, E.S.; Post, J.E.; Heaney, P.J.; Ling, F.T.; Kerisit, S.N. XPS determination of Mn oxidation states in Mn (hydr)oxides. Appl. Surf. Sci. 2016, 366, 475–485. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-S.; Cook, J.B.; Lin, H.; Ko, J.S.; Tolbert, S.H.; Ozolins, V.; Dunn, B. Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3−x. Nat. Mater. 2017, 16, 454. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.; Zhu, C.; Yang, P.; Xia, X.; Liu, J.; Wang, J.; Fan, X.; Savilov, S.V.; Lin, J.; Fan, H.J.; et al. Array of nanosheets render ultrafast and high-capacity Na-ion storage by tunable pseudocapacitance. Nat. Commun. 2016, 7, 12122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, M.; Zhou, W.; Tsai, M.-C.; Zhou, J.; Guan, M.; Lin, M.-C.; Zhang, B.; Hu, Y.; Wang, D.-Y.; Yang, J.; et al. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nat. Commun. 2014, 5, 4695. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, G.; Cui, Y. Nanostructured sulfur cathodes. Chem. Soc. Rev. 2013, 42, 3018–3032. [Google Scholar] [CrossRef]
- Guo, C.; Liu, H.; Li, J.; Hou, Z.; Liang, J.; Zhou, J.; Zhu, Y.; Qian, Y. Ultrathin delta-MnO2 nanosheets as cathode for aqueous rechargeable zinc ion battery. Electrochim. Acta 2019, 304, 370–377. [Google Scholar] [CrossRef]
- Xu, J.-W.; Gao, Q.-L.; Xia, Y.-M.; Lin, X.-S.; Liu, W.-L.; Ren, M.-M.; Kong, F.-G.; Wang, S.-J.; Lin, C. High-performance reversible aqueous zinc-ion battery based on iron-doped alpha-manganese dioxide coated by polypyrrole. J. Colloid Interface Sci. 2021, 598, 419–429. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Wu, X.; Cho, Y.-R. Enhanced Electrochemical Performance of Zn/VOx Batteries by a Carbon-Encapsulation Strategy. ACS Appl. Mater. Interfaces 2022, 14, 11654–11662. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Zhang, L.; Li, J.; Wang, W.; Yang, Z.; Zhang, L.; Wang, Y.; Chen, J.; Huang, Y.; et al. Graphene-like Vanadium Oxygen Hydrate (VOH) Nanosheets Intercalated and Exfoliated by Polyaniline (PANI) for Aqueous Zinc-Ion Batteries (ZIBs). ACS Appl. Mater. Interfaces 2020, 12, 31564–31574. [Google Scholar] [CrossRef]
- Liu, X.; Ma, L.; Du, Y.; Lu, Q.; Yang, A.; Wang, X. Vanadium Pentoxide Nanofibers/Carbon Nanotubes Hybrid Film for High-Performance Aqueous Zinc-Ion Batteries. Nanomaterials 2021, 11, 1054. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shen, X.; Chen, T.; Xu, Q. The spinel MnFe2O4 grown in biomass-derived porous carbons materials for high-performance cathode materials of aqueous zinc-ion batteries. J. Alloys Compd. 2022, 904, 164002. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, J.; Zhu, B.; Zhang, Z.; Xiang, Y.; Tang, C.; Ding, Q.; Wu, X. The Synthesis of Manganese Hydroxide Nanowire Arrays for a High-Performance Zinc-Ion Battery. Nanomaterials 2022, 12, 2514. https://doi.org/10.3390/nano12152514
Gong J, Zhu B, Zhang Z, Xiang Y, Tang C, Ding Q, Wu X. The Synthesis of Manganese Hydroxide Nanowire Arrays for a High-Performance Zinc-Ion Battery. Nanomaterials. 2022; 12(15):2514. https://doi.org/10.3390/nano12152514
Chicago/Turabian StyleGong, Jiangfeng, Bingxin Zhu, Zhupeng Zhang, Yuanyuan Xiang, Chunmei Tang, Qingping Ding, and Xiang Wu. 2022. "The Synthesis of Manganese Hydroxide Nanowire Arrays for a High-Performance Zinc-Ion Battery" Nanomaterials 12, no. 15: 2514. https://doi.org/10.3390/nano12152514