Enhanced Visible Light-Driven Photoelectrocatalytic Degradation of Paracetamol at a Ternary z-Scheme Heterojunction of Bi2WO6 with Carbon Nanoparticles and TiO2 Nanotube Arrays Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of z-Scheme Bi2WO6/CNP/TiO2 NTA
2.3. Characterisation
2.4. Electrochemical and Photoelectrochemical Characterisation
3. Results
3.1. Structural and Morphological Characterisation
3.2. Optical Properties
3.3. Electrochemical Studies
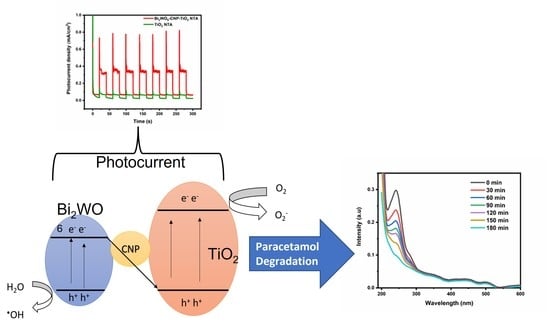
3.4. Photoelectrochemical Studies
3.5. Plausible Mechanism for Paracetamol Degradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olvera-Rodríguez, I.; Hernández, R.; Medel, A.; Guzmán, C.; Escobar-Alarcón, L.; Brillas, E.; Sirés, I.; Esquivel, K. TiO2 /Au/ TiO2 Multilayer Thin-Film Photoanodes Synthesized by Pulsed Laser Deposition for Photoelectrochemical Degradation of Organic Pollutants. Sep. Purif. Technol. 2019, 224, 189–198. [Google Scholar] [CrossRef]
- Högestätt, E.D.; Jönsson, B.A.G.; Ermund, A.; Andersson, D.A.; Björk, H.; Alexander, J.P.; Cravatt, B.F.; Basbaum, A.I.; Zygmunt, P.M. Conversion of Acetaminophen to the Bioactive N-Acylphenolamine AM404 via Fatty Acid Amide Hydrolase-Dependent Arachidonic Acid Conjugation in the Nervous System. J. Biol. Chem. 2005, 280, 31405–31412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López Zavala, M.Á.; Jaber Lara, C.R. Degradation of Paracetamol and Its Oxidation Products in Surface Water by Electrochemical Oxidation. Environ. Eng. Sci. 2018, 35, 1248–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalgic, G.; Turkdogan, F.I.; Yetilmezsoy, K.; Kocak, E. Treatment of Real Paracetamol. Chem. Ind. Chem. Eng. Q 2017, 23, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Fatima, S.; Asif, N.; Ahmad, R.; Fatma, T. Toxicity of NSAID Drug (Paracetamol) to Nontarget Organism—Nostoc Muscorum. Environ. Sci. Pollut. Res. 2020, 27, 35208–35216. [Google Scholar] [CrossRef]
- Macías-García, A.; García-Sanz-Calcedo, J.; Carrasco-Amador, J.P.; Segura-Cruz, R. Adsorption of Paracetamol in Hospital Wastewater through Activated Carbon Filters. Sustainability 2019, 11, 2672. [Google Scholar] [CrossRef] [Green Version]
- Lozano-Morales, S.A.; Morales, G.; López Zavala, M.Á.; Arce-Sarria, A.; Machuca-Martínez, F. Photocatalytic Treatment of Paracetamol Using TiO2 Nanotubes: Effect of PH. Processes 2019, 7, 319. [Google Scholar] [CrossRef] [Green Version]
- Audino, F.; Santamaria, J.M.T.; Del Valle Mendoza, L.J.; Graells, M.; Pérez-Moya, M. Removal of Paracetamol Using Effective Advanced Oxidation Processes. Int. J. Environ. Res. Public Health 2019, 16, 505. [Google Scholar] [CrossRef] [Green Version]
- Ghanbari, F.; Giannakis, S.; Lin, K.Y.A.; Wu, J.; Madihi-Bidgoli, S. Acetaminophen Degradation by a Synergistic Peracetic Acid/UVC-LED/Fe(II) Advanced Oxidation Process: Kinetic Assessment, Process Feasibility and Mechanistic Considerations. Chemosphere 2021, 263, 128119. [Google Scholar] [CrossRef]
- Arotiba, O.A.; Orimolade, B.O.; Koiki, B.A. Visible Light–Driven Photoelectrocatalytic Semiconductor Heterojunction Anodes for Water Treatment Applications. Curr. Opin. Electrochem. 2020, 22, 25–34. [Google Scholar] [CrossRef]
- Orimolade, B.O.; Arotiba, O.A. Towards Visible Light Driven Photoelectrocatalysis for Water Treatment: Application of a FTO/BiVO4/Ag2S Heterojunction Anode for the Removal of Emerging Pharmaceutical Pollutants. Sci. Rep. 2020, 10, 5348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orimolade, B.O.; Koiki, B.A.; Zwane, B.N.; Peleyeju, G.M.; Mabuba, N.; Arotiba, O.A. Interrogating Solar Photoelectrocatalysis on an Exfoliated Graphite-BiVO4/ZnO Composite Electrode towards Water Treatment. RSC Adv. 2019, 9, 16586–16595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nada, A.A.; Orimolade, B.O.; El-Maghrabi, H.H.; Koiki, B.A.; Rivallin, M.; Bekheet, M.F.; Viter, R.; Damberga, D.; Lesage, G.; Iatsunskyi, I.; et al. Photoelectrocatalysis of Paracetamol on Pd–ZnO/N-Doped Carbon Nanofibers Electrode. Appl. Mater. Today 2021, 24, 101129. [Google Scholar] [CrossRef]
- Sharma, S.; Ibhadon, A.O.; Francesconi, M.G.; Mehta, S.K.; Elumalai, S.; Kansal, S.K.; Umar, A.; Baskoutas, S. Bi2 WO6/C-Dots /TiO2: A Novel Z-Scheme Photocatalyst for the Degradation of Fluoroquinolone Levofloxacin from Aqueous Medium. Nanomaterials 2020, 10, 910. [Google Scholar] [CrossRef]
- Hu, K.; Chen, C.; Zhu, Y.; Zeng, G.; Huang, B.; Chen, W.; Liu, S.; Lei, C.; Li, B.; Yang, Y. Ternary Z-Scheme Heterojunction of Bi2WO6 with Reduced Graphene Oxide (RGO) and Meso-Tetra (4-Carboxyphenyl) Porphyrin (TCPP) for Enhanced Visible-Light Photocatalysis. J. Colloid Interface Sci. 2019, 540, 115–125. [Google Scholar] [CrossRef]
- Adhikari, S.; Selvaraj, S.; Kim, D.H. Construction of Heterojunction Photoelectrode via Atomic Layer Deposition of Fe2O3 on Bi2WO6 for Highly Efficient Photoelectrochemical Sensing and Degradation of Tetracycline. Appl. Catal. B Environ. 2019, 244, 11–24. [Google Scholar] [CrossRef]
- Syed, N.; Huang, J.; Feng, Y.; Wang, X.; Cao, L. Carbon-Based Nanomaterials via Heterojunction Serving as Photocatalyst. Front. Chem. 2019, 7, 713. [Google Scholar] [CrossRef]
- Rana, A.; Kumar, A.; Sharma, G.; Naushad, M.; Bathula, C.; Stadler, F.J. Pharmaceutical Pollutant as Sacrificial Agent for Sustainable Synergistic Water Treatment and Hydrogen Production via Novel Z- Scheme Bi7O9I3/B4C Heterojunction Photocatalysts. J. Mol. Liq. 2021, 343, 117652. [Google Scholar] [CrossRef]
- Chaplin, B.P. Critical Review of Electrochemical Advanced Oxidation Processes for Water Treatment Applications. Environ. Sci. Process. Impacts 2014, 16, 1182–1203. [Google Scholar] [CrossRef]
- Velempini, T.; Prabakaran, E.; Pillay, K. Recent Developments in the Use of Metal Oxides for Photocatalytic Degradation of Pharmaceutical Pollutants in Water—A Review. Mater. Today Chem. 2021, 19, 100380. [Google Scholar] [CrossRef]
- Borràs-Ferrís, J.; Sánchez-Tovar, R.; Blasco-Tamarit, E.; Muñoz-Portero, M.J.; Fernández-Domene, R.M.; García-Antón, J. TiO2 Nanostructures for Photoelectrocatalytic Degradation of Acetaminophen. Nanomaterials 2019, 9, 583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koiki, B.A.; Orimolade, B.O.; Zwane, B.N.; Nkosi, D.; Mabuba, N.; Arotiba, O.A. Cu2O on Anodised TiO2 Nanotube Arrays: A Heterojunction Photoanode for Visible Light Assisted Electrochemical Degradation of Pharmaceuticals in Water. Electrochim. Acta 2020, 340, 135944. [Google Scholar] [CrossRef]
- Cheng, T.; Gao, H.; Liu, G.; Pu, Z.; Wang, S.; Yi, Z.; Yang, H. Preparation of core-shell heterojunction photocatalysts by coating CdS nanoparticles onto Bi4Ti3O12 hierarchical microspheres and their photocatalytic removal of organic pollutants and Cr (VI) ions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127918. [Google Scholar] [CrossRef]
- Umukoro, E.H.; Peleyeju, M.G.; Idris, A.O.; Ngila, J.C.; Mabuba, N.; Rhyman, L.; Ramasami, P.; Arotiba, O.A. Photoelectrocatalytic application of palladium decorated zinc oxide-expanded graphite electrode for the removal of 4-nitrophenol: Experimental and computational studies. RSC Adv. 2018, 8, 10255–10266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khedr, T.M.; Wang, K.; Kowalski, D.; El-Sheikh, S.M.; Abdeldayem, H.M.; Ohtani, B.; Kowalska, E. Bi2WO6-based Z-scheme photocatalysts: Principles, mechanisms and photocatalytic applications. J. Environ. Chem. Eng. 2022, 10, 107838. [Google Scholar] [CrossRef]
- Peleyeju, M.G.; Arotiba, O.A. Recent trend in visible-light photoelectrocatalytic systems for degradation of organic contaminants in water/wastewater. Environ. Sci. Water Res. Technol. 2018, 4, 1389–1411. [Google Scholar] [CrossRef]
- Tshwenya, L.; Arotiba, O.A. Ethylenediamine Functionalized Carbon Nanoparticles: Synthesis, Characterization, and Evaluation for Cadmium Removal from Water. RSC Adv. 2017, 7, 34226–34235. [Google Scholar] [CrossRef] [Green Version]
- Bernal, V.; Erto, A.; Giraldo, L.; Moreno-Piraján, J.C. Effect of Solution PH on the Adsorption of Paracetamol on Chemically Modified Activated Carbons. Molecules 2017, 22, 1032. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahhumane, N.; Cele, L.M.; Muzenda, C.; Nkwachukwu, O.V.; Koiki, B.A.; Arotiba, O.A. Enhanced Visible Light-Driven Photoelectrocatalytic Degradation of Paracetamol at a Ternary z-Scheme Heterojunction of Bi2WO6 with Carbon Nanoparticles and TiO2 Nanotube Arrays Electrode. Nanomaterials 2022, 12, 2467. https://doi.org/10.3390/nano12142467
Mahhumane N, Cele LM, Muzenda C, Nkwachukwu OV, Koiki BA, Arotiba OA. Enhanced Visible Light-Driven Photoelectrocatalytic Degradation of Paracetamol at a Ternary z-Scheme Heterojunction of Bi2WO6 with Carbon Nanoparticles and TiO2 Nanotube Arrays Electrode. Nanomaterials. 2022; 12(14):2467. https://doi.org/10.3390/nano12142467
Chicago/Turabian StyleMahhumane, Nondumiso, Leskey M. Cele, Charles Muzenda, Oluchi V. Nkwachukwu, Babatunde A. Koiki, and Omotayo A. Arotiba. 2022. "Enhanced Visible Light-Driven Photoelectrocatalytic Degradation of Paracetamol at a Ternary z-Scheme Heterojunction of Bi2WO6 with Carbon Nanoparticles and TiO2 Nanotube Arrays Electrode" Nanomaterials 12, no. 14: 2467. https://doi.org/10.3390/nano12142467