First-Principles Study of Irn (n = 3–5) Clusters Adsorbed on Graphene and Hexagonal Boron Nitride: Structural and Magnetic Properties
Abstract
:1. Introduction
2. Materials and Methods
3. Results
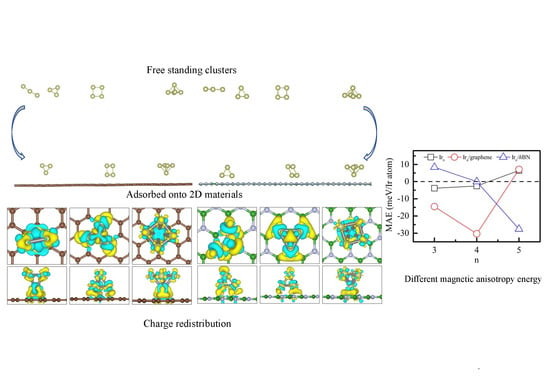
3.1. Structural Properties
3.2. Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Douglass, D.C.; Bucher, J.P.; Bloomfield, L.A. Magnetic studies of free nonferromagnetic clusters. Phys. Rev. B 1992, 45, 6341–6344. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.J.; Louderback, J.G.; Bloomfield, L.A. Experimental observation of magnetism in rhodium clusters. Phys. Rev. Lett. 1993, 71, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.J.; Louderback, J.G.; Apsel, S.E.; Bloomfield, L.A. Magnetism in 4d-transition metal clusters. Phys. Rev. B 1994, 49, 12295–12298. [Google Scholar] [CrossRef] [PubMed]
- Knight, W.D.; Clemenger, K.; de Heer, W.A.; Saunders, W.A.; Chou, M.Y.; Cohen, M.L. Electronic Shell Structure and Abundances of Sodium Clusters. Phys. Rev. Lett. 1984, 52, 2141–2143. [Google Scholar] [CrossRef]
- Chaves, A.S.; Piotrowski, M.J.; Da Silva, J.L.F. Evolution of the structural, energetic, and electronic properties of the 3d, 4d, and 5d transition-metal clusters (30 TMn systems for n = 2–15): A density functional theory investigation. Phys. Chem. Chem. Phys. 2017, 19, 15484–15502. [Google Scholar] [CrossRef] [PubMed]
- Gaston, N.; Schwerdtfeger, P. From the van der Waals dimer to the solid state of mercury with relativisticab initioand density functional theory. Phys. Rev. B 2006, 74, 024105. [Google Scholar] [CrossRef]
- Song, W.; Lu, W.-C.; Wang, C.Z.; Ho, K.M. Magnetic and electronic properties of the nickel clusters Nin (n ≤ 30). Comput. Theor. Chem. 2011, 978, 41–46. [Google Scholar] [CrossRef]
- Ruiz-Díaz, P.; Ricardo-Chávez, J.L.; Dorantes-Dávila, J.; Pastor, G.M. Magnetism of small Cr clusters: Interplay between structure, magnetic order, and electron correlations. Phys. Rev. B 2010, 81, 224431. [Google Scholar] [CrossRef]
- Yuan, H.K.; Chen, H.; Ahmed, A.S.; Zhang, J.F. Density-functional study of Scn (n = 2–16) clusters: Lowest-energy structures, electronic structure, and magnetism. Phys. Rev. B 2006, 74, 144434. [Google Scholar] [CrossRef]
- Datta, S.; Kabir, M.; Saha-Dasgupta, T. Ab initio study of structural stability of small 3d late transition metal clusters: Interplay of magnetization and hybridization. Phys. Rev. B 2011, 84, 075429. [Google Scholar] [CrossRef]
- Du, J.; Sun, X.; Chen, J.; Jiang, G. A Theoretical Study on Small Iridium Clusters: Structural Evolution, Electronic and Magnetic Properties, and Reactivity Predictors. J. Phys. Chem. A 2010, 114, 12825–12833. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zheng, J.-M.; Zhao, P.; Zheng, L.-L.; Ren, Z.-Y. The relativistic density functional investigations on geometries, electronic and magnetic properties of Irn (n = 1–13) clusters. Chin. Phys. B 2010, 19, 083601. [Google Scholar]
- Ilgaz Aysan, I.; Gorkan, T.; Ozdemir, I.; Kadioglu, Y.; Gokoglu, G.; Akturk, E. Electronic structure, cohesive and magnetic properties of iridium oxide clusters adsorbed on graphene. J. Mol. Graph. Model. 2020, 101, 107726. [Google Scholar] [CrossRef]
- Niemeyer, M.; Hirsch, K.; Zamudio-Bayer, V.; Langenberg, A.; Vogel, M.; Kossick, M.; Ebrecht, C.; Egashira, K.; Terasaki, A.; Moller, T.; et al. Spin coupling and orbital angular momentum quenching in free iron clusters. Phys. Rev. Lett. 2012, 108, 057201. [Google Scholar] [CrossRef]
- Kumar, V.; Kawazoe, Y. Icosahedral growth, magnetic behavior, and adsorbate-induced metal-nonmetal transition in palladium clusters. Phys. Rev. B 2002, 66, 144413. [Google Scholar] [CrossRef]
- De Oliveira, A.Z.; Jorge, F.E. Structural, electronic, electrical, and magnetic properties of Rh (1 ≤ n ≤ 13) clusters. Comput. Theor. Chem. 2020, 1177, 112765. [Google Scholar] [CrossRef]
- Jorge, F.E.; da Costa Venâncio, J.R. Structure, stability, catalytic activity, and polarizabilities of small iridium clusters. Chin. Phys. B 2018, 27, 063102. [Google Scholar] [CrossRef]
- Yuan, H.K.; Chen, H.; Kuang, A.L.; Tian, C.L.; Wang, J.Z. The spin and orbital moment of Fe(n) (n = 2–20) clusters. J. Chem. Phys. 2013, 139, 034314. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Chang, C.M.; Hsing, C.R. Puzzle of magnetic moments of Ni clusters revisited using quantum Monte Carlo method. J. Chem. Phys. 2017, 146, 084313. [Google Scholar] [CrossRef]
- Langenberg, A.; Hirsch, K.; Ławicki, A.; Zamudio-Bayer, V.; Niemeyer, M.; Chmiela, P.; Langbehn, B.; Terasaki, A.; Issendorff, B.V.; Lau, J.T. Spin and orbital magnetic moments of size-selected iron, cobalt, and nickel clusters. Phys. Rev. B 2014, 90, 184420. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Kawazoe, Y. Evolution of atomic and electronic structure of Pt clusters: Planar, layered, pyramidal, cage, cubic, and octahedral growth. Phys. Rev. B 2008, 77, 205418. [Google Scholar] [CrossRef]
- Liang, X.; Wu, X.; Huang, X.; Su, Y.; Hu, J.; Zhao, J. Magnetic Anisotropy of Small Irn Clusters (n = 2–5). J. Cluster. Sci. 2016, 27, 935–946. [Google Scholar] [CrossRef]
- Bernareggi, M.; Chiarello, G.L.; West, G.; Ratova, M.; Ferretti, A.M.; Kelly, P.; Selli, E. Cu and Pt clusters deposition on TiO2 powders by DC magnetron sputtering for photocatalytic hydrogen production. Catal. Today 2019, 326, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Lu, S.-J.; Liang, X.; Su, Y.; Sai, L.; Zhang, Z.-G.; Zhao, J.; Xu, H.-G.; Zheng, W. Structures and Electronic Properties of V3Sin– (n = 3–14) Clusters: A Combined Ab Initio and Experimental Study. J. Phys. Chem. C 2015, 119, 10987–10994. [Google Scholar] [CrossRef]
- Liang, X.-Q.; Deng, X.-J.; Lu, S.-J.; Huang, X.-M.; Zhao, J.-J.; Xu, H.-G.; Zheng, W.-J.; Zeng, X. Probing Structural, Electronic, and Magnetic Properties of Iron-Doped Semiconductor Clusters Fe2Gen−/0 (n = 3–12) via Joint Photoelectron Spectroscopy and Density Functional Study. J. Phys. Chem. C 2017, 121, 7037–7046. [Google Scholar] [CrossRef]
- Huang, X.; Xu, H.G.; Lu, S.; Su, Y.; King, R.B.; Zhao, J.; Zheng, W. Discovery of a silicon-based ferrimagnetic wheel structure in VxSi12 (x = 1–3) clusters: Photoelectron spectroscopy and density functional theory investigation. Nanoscale 2014, 6, 14617–14621. [Google Scholar] [CrossRef]
- Barker, B.A.; Bradley, A.J.; Ugeda, M.M.; Coh, S.; Zettl, A.; Crommie, M.F.; Louie, S.G.; Cohen, M.L. Geometry and electronic structure of iridium adsorbed on graphene. Phys. Rev. B 2019, 99, 075431. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, C.Z.; Lin, H.Q.; Hupalo, M.; Thiel, P.A.; Ho, K.M.; Tringides, M.C. Structures and magnetic properties of Fe clusters on graphene. Phys. Rev. B 2014, 90, 155444. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.-Z. Growth mode and structures of magnetic Mn clusters on graphene. RSC Adv. 2016, 6, 64595–64604. [Google Scholar] [CrossRef]
- Cui, H.; Jia, P. Doping effect of small Rhn (n = 1–4) clusters on the geometric and electronic behaviors of MoS2 monolayer: A first-principles study. Appl. Surf. Sci. 2020, 526, 146659. [Google Scholar] [CrossRef]
- Ju, W.; Li, T.; Su, X.; Li, H.; Li, X.; Ma, D. Au cluster adsorption on perfect and defective MoS2 monolayers: Structural and electronic properties. Phys. Chem. Chem. Phys. 2017, 19, 20735–20748. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, Y.; Kang, L. Stability and nucleation of Irn (n = 1–5) clusters on different γ-Al2O3 surfaces: A density functional theory study. Phys. Lett. A 2016, 380, 718–725. [Google Scholar] [CrossRef]
- Chen, Y.; Huo, M.; Chen, T.; Li, Q.; Sun, Z.; Song, L. The properties of Irn (n = 2–10) clusters and their nucleation on gamma-Al2O3 and MgO surfaces: From ab initio studies. Phys. Chem. Chem. Phys. 2015, 17, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, H.; Hu, J.; Wu, R.Q. Search for giant magnetic anisotropy in transition-metal dimers on defected hexagonal boron nitride sheet. J. Chem. Phys. 2016, 144, 204704. [Google Scholar] [CrossRef]
- Xu, M.; Liang, T.; Shi, M.; Chen, H. Graphene-like two-dimensional materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef]
- Guo, M.; Liang, X.; Wang, H.; Zhang, J. Magnetic anisotropy of iridium dimers on two-dimensional materials. Phys. Chem. Chem. Phys. 2020, 22, 238–244. [Google Scholar] [CrossRef]
- Cahangirov, S.; Topsakal, M.; Aktürk, E.; Şahin, H.; Ciraci, S. Two-and one-dimensional honeycomb structures of silicon and germanium. Phy. Rev. Lett. 2009, 102, 236804. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [Green Version]
- Ge, G.-X.; Yan, H.-X.; Yang, J.-M.; Zhou, L.; Wan, J.-G.; Zhao, J.-J.; Wang, G.-H. Manipulation of magnetic anisotropy in Irn+1 clusters by Co atom. Phys. A 2016, 453, 194–202. [Google Scholar] [CrossRef]
- Yong, Y.; Hao, X.; Li, C.; Li, X.; Li, T.; Cui, H.; Lv, S. Density functional studies of small silicon clusters adsorbed on graphene. RSC Adv. 2015, 5, 38680–38689. [Google Scholar] [CrossRef]
- Ostad, F.Z.; Ghazi, M.E.; Javan, M.; Izadifard, M. DFT study of Ptn, Pdn, and Irn (n = 5, 6) clusters adsorbed on graphene: Structural and electronic properties. Phys. B 2019, 575, 411678. [Google Scholar] [CrossRef]
- Zargari, F.; Javan, M.; Ghazi, M.E.; Izadifard, M. Graphene-like boron nitride supported Irn, Pdn, and Ptn (n = 5, 6) clusters: A DFT study. Diam. Relat. Mater. 2020, 110, 108110. [Google Scholar] [CrossRef]
- Tang, W.; Sanville, E.; Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 2009, 21, 084204. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.S.; Wu, R.; Freeman, A.J. First-principles theory of surface magnetocrystalline anisotropy and the diatomic-pair model. Phys. Rev. B 1993, 47, 14932–14947. [Google Scholar] [CrossRef]








| ΔE | Eb | d | μ | μtot | δ | |
|---|---|---|---|---|---|---|
| Ir3b/graphene-H | 0 | 5.352 | 1.771 | 1.408 | 1.287 | −0.163 |
| Ir3b/graphene-BH | 0.159 | 5.192 | 2.007 | 2.385 | 2.517 | −0.221 |
| Ir3a/graphene-HH | 0.278 | 4.950 | 1.714 | 1.017 | 1.083 | −0.269 |
| Ir3b/hBN-TT | 0 | 5.583 | 2.248 | 2.687 | 2.713 | −0.0305 |
| Ir3b/hBN-H | 0.268 | 5.315 | 1.957 | 2.644 | 2.702 | 0.101 |
| Ir3a/hBN-T | 0.691 | 4.768 | 2.253 | 0.822 | 0.829 | 0.107 |
| Ir4a/graphene-BB | 0 | 4.818 | 2.662 | 4.929 | 4.973 | −0.245 |
| Ir4a/graphene-T | 0.093 | 4.725 | 2.080 | 4.943 | 4.941 | −0.104 |
| Ir4b/graphene-H | 0.462 | 5.607 | 1.895 | 0.548 | 0.259 | −0.037 |
| Ir4a/hBN-TT | 0 | 5.546 | 2.363 | 1.535 | 1.519 | −0.023 |
| Ir4a/hBN-T | 0.411 | 5.135 | 2.241 | 2.877 | 2.900 | 0.010 |
| Ir4c/hBN-TTT | 0.938 | 6.722 | 2.075 | 0 | 0 | −0.133 |
| Ir5a/graphene-H | 0 | 5.434 | 1.989 | 4.516 | 4.539 | −0.173 |
| Ir5a/graphene-TTTT | 0.586 | 4.847 | 2.374 | 2.181 | 2.196 | −0.468 |
| Ir5b/graphene-BB | 0.645 | 5.029 | 2.595 | 2.452 | 2.452 | −0.170 |
| Ir5a/hBN-H | 0 | 5.554 | 1.736 | 3.861 | 3.844 | 0.093 |
| Ir5c/hBN-T | 0.103 | 6.567 | 2.298 | 2.377 | 2.358 | 0 |
| Ir5b/hBN-H | 0.172 | 5.622 | 1.742 | 3.940 | 3.933 | 0.067 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, M.; Chu, L.; Guo, M.; Su, Y.; Zhang, J. First-Principles Study of Irn (n = 3–5) Clusters Adsorbed on Graphene and Hexagonal Boron Nitride: Structural and Magnetic Properties. Nanomaterials 2022, 12, 2436. https://doi.org/10.3390/nano12142436
Ge M, Chu L, Guo M, Su Y, Zhang J. First-Principles Study of Irn (n = 3–5) Clusters Adsorbed on Graphene and Hexagonal Boron Nitride: Structural and Magnetic Properties. Nanomaterials. 2022; 12(14):2436. https://doi.org/10.3390/nano12142436
Chicago/Turabian StyleGe, Mei, Leiting Chu, Miaomiao Guo, Yan Su, and Junfeng Zhang. 2022. "First-Principles Study of Irn (n = 3–5) Clusters Adsorbed on Graphene and Hexagonal Boron Nitride: Structural and Magnetic Properties" Nanomaterials 12, no. 14: 2436. https://doi.org/10.3390/nano12142436