Self-Supported Co3O4@Mo-Co3O4 Needle-like Nanosheet Heterostructured Architectures of Battery-Type Electrodes for High-Performance Asymmetric Supercapacitors
Abstract
:1. Introduction
2. Experiment Procedure
2.1. Chemical Details
2.2. Synthesis of Co3O4 and Co3O4@Mo-Co3O4
2.3. Characterizations
2.4. Asymmetric Supercapacitor Preparation
3. Results and Discussion
3.1. Electrochemical Properties of Electrode Materials
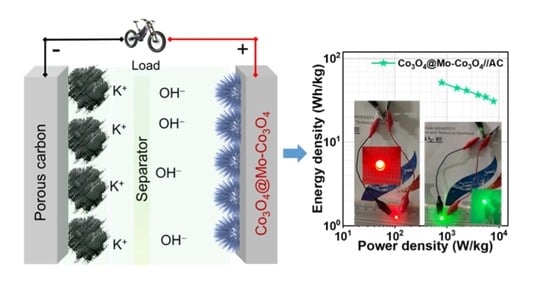
3.2. Electrochemical Properties of a Co3O4@Mo-Co3O4//AC Device
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoon, J.H.; Kumar, Y.A.; Sambasivam, S.; Hira, S.A.; Krishna, T.N.V.; Zeb, K.; Uddin, W.; Kumar, K.D.; Obaidat, I.M.; Kim, S.; et al. Highly efficient copper-cobalt sulfide nano-reeds array with simplistic fabrication strategy for battery-type supercapacitors. J. Energy Storage 2020, 32, 101988. [Google Scholar] [CrossRef]
- Mun, C.H.; Gopi, C.V.V.M.; Vinodh, R.; Sambasivam, S.; Obaidat, I.M.; Kim, H.J. Microflower-like nickel sulfide-lead sulfide hierarchical composites as bind-er-free electrodes for high-performance supercapacitors. J. Energy Storage 2019, 26, 100925. [Google Scholar] [CrossRef]
- Das, H.T.; Mahendraprabhu, K.; Maiyalagan, T.; Elumalai, P. Performance of Solid-state Hybrid Energy-storage Device using Reduced Graphene-oxide Anchored Sol-gel Derived Ni/NiO Nanocomposite. Sci. Rep. 2017, 7, 15342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duraisamy, E.; Das, H.T.; Sharma, A.S.; Elumalai, P. Supercapacitor and photocatalytic performances of hydrothermally-derived Co3O4/CoO@carbon nanocomposite. New J. Chem. 2018, 42, 6114–6124. [Google Scholar] [CrossRef]
- Yedluri, A.K.; Kim, H.-J. Wearable super-high specific performance supercapacitors using a honeycomb with folded silk-like composite of NiCo2O4 nanoplates decorated with NiMoO4 honeycombs on nickel foam. Dalton Trans. 2018, 47, 15545–15554. [Google Scholar] [CrossRef]
- Yedluri, A.K.; Anitha, T.; Kim, H.-J. Fabrication of Hierarchical NiMoO4/NiMoO4 Nanoflowers on Highly Conductive Flexible Nickel Foam Substrate as a Capacitive Electrode Material for Supercapacitors with Enhanced Electrochemical Performance. Energies 2019, 12, 1143. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Khan, A.J.; Arshad, M.; Javed, M.S.; Ahmad, A.; Ahmad Shah, S.S.; Khan, M.R.; Akram, S.; Zulfiqar; Ali, S.; et al. Charge storage in binder-free 2D-hexagonal CoMoO4 nanosheets as a redox active material for pseudocapacitors. Ceram. Int. 2021, 47, 8659–8667. [Google Scholar] [CrossRef]
- Li, P.; Ruan, C.; Xu, J.; Xie, Y. Supercapacitive performance of CoMoO4 with oxygen vacancy porous nanosheet. Electrochim. Acta 2020, 330, 135334. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Durai, G.; Tatarchuk, T.; Sumathi, M.; Kuppusami, P.; Qin, J.; Choi, M.Y. Synthesis of hierarchical structured rare earth metal–doped Co3O4 by polymer combustion method for high performance electrochemical supercapacitor electrode materials. Ionics 2020, 26, 2051–2061. [Google Scholar] [CrossRef]
- Gopi, C.V.V.M.; Vinodh, R.; Sambasivam, S.; Obaidat, I.M.; Kalla, R.M.N.; Kim, H.J. One-pot synthesis of copper oxide–cobalt oxide core–shell nanocactus-like het-erostructures as binder-free electrode materials for high-rate hybrid superca-pacitors. Mater. Today Energy 2019, 14, 100358. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Sambasivam, S.; Hira, S.A.; Zeb, K.; Uddin, W.; Krishna, T.N.V.; Kumar, K.D.; Obaidat, I.M.; Kim, H.J. Boosting the energy density of highly efficient flexible hybrid supercapacitors via selective integration of hierarchical nanostructured energy materials. Electrochim. Acta 2020, 364, 137318. [Google Scholar] [CrossRef]
- Shen, H.; Yang, X.; Song, J.; Gao, H.; Wu, Z.; Yu, J.; Lei, W.; Yang, J.; He, G.; Hao, Q. Electrodeposited molybdenum-doped Co3O4 nanosheet arrays for high-performance and stable hybrid supercapacitors. J. Solid State Electrochem. 2022, 26, 353–363. [Google Scholar] [CrossRef]
- Xia, X.; Lei, W.; Hao, Q.; Wang, W.; Wang, X. One-step synthesis of CoMoO4/graphene composites with enhanced electrochemical properties for supercapacitors. Electrochim. Acta 2013, 99, 253–261. [Google Scholar] [CrossRef]
- Lin, L.; Li, L.; Hussain, S.; Zhao, S.; Wu, L.; Peng, X.; Hu, N. Hierarchical 3D NiCo2O4@ZnWO4 core-shell structures as binder-free electrodes for all-solid-state supercapacitors. Appl. Surf. Sci. 2018, 452, 113–122. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; Banerjee, A.N.; Nallapureddy, R.R.; Joo, S.W. Urea-assisted hydrothermal synthesis of MnMoO4/MnCO3 hybrid electrochemical electrode and fabrication of high-performance asymmetric supercapacitor. J. Mater. Sci. Technol. 2022, 96, 332–344. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Yuan, X.; Yang, Z.; Zhang, M.; Meng, A.; Li, Q. A High-Energy Density Asymmetric Supercapacitor Based on Fe2O3 Nanoneedle Arrays and NiCo2O4/Ni(OH)2 Hybrid Nanosheet Arrays Grown on SiC Nanowire Networks as Free-Standing Advanced Electrodes. Adv. Energy Mater. 2018, 8, 1702787. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; Gaddam, N.; Banerjee, A.N.; Nallapureddy, R.R.; Kumar, Y.A.; Joo, S.W. Facile construction and controllable design of CoTiO3@Co3O4/NCNO hybrid heterojunction nanocomposite electrode for high-performance supercapacitors. Electrochim. Acta 2022, 407, 139868. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Kim, H.-J. Preparation and electrochemical performance of NiCo2O4@NiCo2O4 composite nanoplates for high performance supercapacitor applications. New J. Chem. 2018, 42, 19971–19978. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; Kumar, Y.A.; Mani, G.; Nallapureddy, R.R.; Parvathala, A.; Albaqami, M.D.; Karami, A.M.; Joo, S.W. A novel hybridized needle-like Co3O4/N-CNO composite for superior energy storage asymmetric supercapacitors. J. Alloys Compd. 2022, 908, 164447. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; Anil Kumar, Y.; Nallapureddy, R.R.; Goli, H.R.; Narayan Banerjee, A.; Joo, S.W. In-situ design of porous vanadium nitride@carbon nanobelts: A promising material for high-performance asymmetric supercapacitors. Appl. Surf. Sci. 2022, 575, 151734. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; Nallapureddy, J.; Nallapureddy, R.R.; Neelima, G.; Yedluri, A.K.; Mandal, T.K.; Pejjai, B.; Joo, S.W. Self-assembled and highly faceted growth of Mo and V doped ZnO nanoflowers for high-performance supercapacitors. J. Alloys Compd. 2021, 886, 161234. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, Y.; Sun, G.; Jia, T.; Jia, L.; Zhang, F.; Lin, L.; Zhang, B.; Cao, J.; Zhang, Z. Carbon Nitride Decorated Ball-Flower like Co3O4 Hybrid Composite: Hydrothermal Synthesis and Ethanol Gas Sensing Application. Nanomaterials 2018, 8, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farhadi, S.; Pourzare, K.; Sadeghinejad, S. Simple preparation of ferromagnetic Co3O4 nanoparticles by thermal dissociation of the [CoII(NH3)6](NO3)2 complex at low temperature. J. Nanostruct. Chem. 2013, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhu, G.; Ge, B.; Zhou, H.; Yuan, A.; Shen, X. Concave Co3O4 octahedral mesocrystal: Polymer -mediated synthesis and sensing properties. CrystEngComm 2012, 14, 6264–6270. [Google Scholar] [CrossRef]
- Mariammal, R.N.; Ramachandran, K.; Kalaiselvan, G.; Arumugam, S.; Renganathan, B.; Sastikumar, D. Effect of magnetism on the ethanol sensitivity of undoped and Mn-doped CuO nanoflakes. Appl. Surf. Sci. 2013, 270, 545–552. [Google Scholar] [CrossRef]
- Aghazadeh, M. Electrochemical preparation and properties of nanostructured Co3O4 as supercapacitor material. J. Appl. Electrochem. 2012, 42, 89–94. [Google Scholar] [CrossRef]
- Khalil, T.E.; Soliman, S.M.; Khalil, N.A.; El-Faham, A.; Foro, S.; El-Dissouky, A. Synthesis, structure, X-ray photoelectron spectroscopy (XPS), and antimicrobial, anticancer, and antioxidant activities of Co (III) complexes based on the antihypertensive hydralazine. Appl. Organomet. Chem. 2022, 36, e6565. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Zhao, J.; Zhao, S.; Zhou, J.; Chen, C.; Tao, K.; Liu, R.; Han, L. Ultrathin nanosheet-assembled hollow microplate CoMoO4 array derived from metal-organic framework for supercapacitor with ultrahigh areal capacitance. J. Power Sources 2019, 430, 51–59. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Liao, F.; Han, X.; Zhang, Y.; Xu, C.; Gao, L. Uniform and porous Mn-doped Co3O4 microspheres: Solvothermal synthesis and their superior supercapacitor performances. Ceram. Int. 2019, 45, 11876–11882. [Google Scholar] [CrossRef]
- Li, G.; Chen, M.; Ouyang, Y.; Yao, D.; Lu, L.; Wang, L.; Xia, X.; Lei, W.; Chen, S.-M.; Mandler, D.; et al. Manganese doped Co3O4 mesoporous nanoneedle array for long cycle-stable supercapacitors. Appl. Surf. Sci. 2019, 469, 941–950. [Google Scholar] [CrossRef]
- Niknam, E.; Naffakh-Moosavy, H.; Moosavifard, S.E.; Afshar, M.G. Multi-shelled bimetal V-doped Co3O4 hollow spheres derived from metal organic framework for high performance supercapacitors. J. Energy Storage 2021, 44, 103508. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, Q.; Xu, M.; Zhai, Q.; Zhang, C. Two-for-one strategy: Three-dimensional porous Fe-doped Co3O4 cathode and N-doped carbon anode derived from a single bimetallic metal-organic framework for enhanced hybrid supercapacitor. J. Colloid Interface Sci. 2021, 583, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Khalid, N.R.; Niaz, N.A.; Nabi, G.; Tahir, M.B.; Rafique, M. Novel Cr and Sn co-doped Co3O4 polygon-based electrode material for supercapacitor application. J. Mater. Sci. Mater. Electron. 2021, 32, 11467–11477. [Google Scholar] [CrossRef]
- Ali, F.; Khalid, N.R.; Nabi, G.; Ul-Hamid, A.; Ikram, M. Hydrothermal synthesis of cerium-doped Co3O4 nanoflakes as electrode for supercapacitor application. Int. J. Energy Res. 2021, 45, 1999–2010. [Google Scholar] [CrossRef]






| Unit Cell Parameters | |
|---|---|
| a, (Å) | 8.08 |
| b, (Å) | 8.08 |
| c, (Å) | 8.08 |
| α, (°) | 90 |
| β, (°) | 90 |
| γ, (°) | 90 |
| Cell volume (Å)3 | 528.24 |
| Density (g/cm3) | 6.05 |
| Crystal system and Space group number | Cubic & (Fd-3m, 227) |
| Structure parameters | |
| Atoms | 130 |
| Bonds | 168 |
| Polyhedra | 34 |
| Refinement parameters | |
| Rp | 1.762 |
| Rwp | 2.216 |
| Goodness of fit (GOF) | 0.805 |
| R-Structure factor | 4.68 |
| R-Bragg factor | 6.89 |
| Element | X | Y | Z | Occupancy | Site | Sym. |
|---|---|---|---|---|---|---|
| O1 | 0.14000 | 0.14000 | 0.14000 | 1.000 | 16e | 0.3 m |
| O2 | 0.61000 | 0.61000 | 0.61000 | 1.000 | 16e | 0.3 m |
| Co1 | 0.75000 | 0.75000 | 0.75000 | 1.000 | 4d | −43 m |
| Co2 | 0.00000 | 0.00000 | 0.00000 | 1.000 | 4a | −43 m |
| Co3 | 0.37500 | 0.37500 | 0.37500 | 1.000 | 16e | 0.3 m |
| Electrode | Electrolyte | Specific Capacitance | Retention Rate | Energy Density (Wh kg−1) | Power Density (W kg−1) | Ref. |
|---|---|---|---|---|---|---|
| Mn@Co3O4 | 2M KOH | 773 F g−1 | 73.9%/5000 cycles | NA | NA | [29] |
| Mn-Co3O4/NF | 2M KOH | 668.4 F g−1 | 104%/10,000 | 10.63 | 14,700 | [30] |
| V-Co3O4 | 3M KOH | 1593 F g−1 | NA | 66.88 | 240 | [31] |
| Fe-Co3O4 | 6M KOH | 767.9 C g−1 | 90%/4000 | 37 | 750 | [32] |
| Cr, Sn- Co3O4 | 3M KOH | 1413.56 Fg−1 | 89.41%/3000 | NA | NA | [33] |
| Ce-Co3O4 | 3M KOH | 1309.6 F g−1 | 90.86%/2000 | NA | NA | [34] |
| Mo-Co3O4 | 2M KOH | 1850 F g−1 | 90%/4000 | -- | -- | This Work |
| Mo-Co3O4//AC | 2M KOH | 148 F g−1 | 89%/4000 | 51.4 | 790 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, Y.A.; Das, H.T.; Guddeti, P.R.; Nallapureddy, R.R.; Pallavolu, M.R.; Alzahmi, S.; Obaidat, I.M. Self-Supported Co3O4@Mo-Co3O4 Needle-like Nanosheet Heterostructured Architectures of Battery-Type Electrodes for High-Performance Asymmetric Supercapacitors. Nanomaterials 2022, 12, 2330. https://doi.org/10.3390/nano12142330
Kumar YA, Das HT, Guddeti PR, Nallapureddy RR, Pallavolu MR, Alzahmi S, Obaidat IM. Self-Supported Co3O4@Mo-Co3O4 Needle-like Nanosheet Heterostructured Architectures of Battery-Type Electrodes for High-Performance Asymmetric Supercapacitors. Nanomaterials. 2022; 12(14):2330. https://doi.org/10.3390/nano12142330
Chicago/Turabian StyleKumar, Yedluri Anil, Himadri Tanaya Das, Phaneendra Reddy Guddeti, Ramesh Reddy Nallapureddy, Mohan Reddy Pallavolu, Salem Alzahmi, and Ihab M. Obaidat. 2022. "Self-Supported Co3O4@Mo-Co3O4 Needle-like Nanosheet Heterostructured Architectures of Battery-Type Electrodes for High-Performance Asymmetric Supercapacitors" Nanomaterials 12, no. 14: 2330. https://doi.org/10.3390/nano12142330