Controlled Synthesis of Platinum and Silver Nanoparticles Using Multivalent Ligands
Abstract
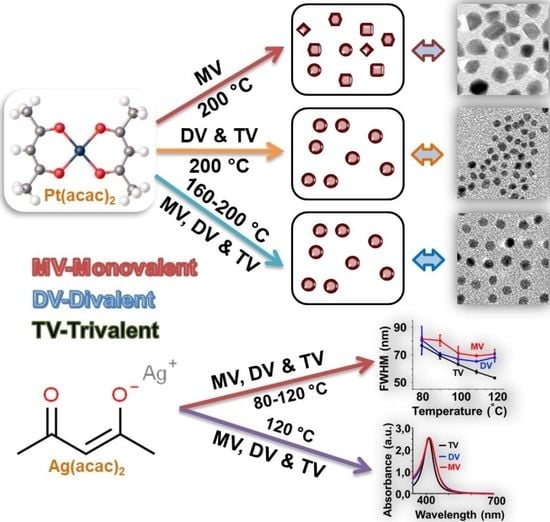
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Synthesis of Multivalent Ligands
2.3. Synthesis of Amine-Coated PtNPs and AgNPs at Different Temperatures by Performing a Stepwise Process
2.4. Synthesis of Amine-Coated PtNPs by Performing a One-Step Process
2.5. Characterization of the Samples
3. Results and Discussion
3.1. Temperature and Ligand Effects on the Formation of PtNPs and AgNPs by Performing the Stepwise Process
3.2. Effects of Mono- and Multivalent Amine Ligands on the Formation of PtNPs and AgNPs by a One-Step Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Narayan, N.; Meiyazhagan, A.; Vajtai, R. Metal Nanoparticles as Green Catalysts. Materials 2019, 12, 3602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, C.; Lyu, F.; Yin, Y. Encapsulated Metal Nanoparticles for Catalysis. Chem. Rev. 2021, 121, 834–881. [Google Scholar] [CrossRef] [PubMed]
- Sápi, A.; Rajkumar, T.; Kiss, J.; Kukovecz, Á.; Kónya, Z.; Somorjai, G.A. Metallic Nanoparticles in Heterogeneous Catalysis. Catal. Lett. 2021, 151, 2153–2175. [Google Scholar] [CrossRef]
- Ndolomingo, M.J.; Bingwa, N.; Meijboom, R. Review of supported metal nanoparticles: Synthesis methodologies, advantages and application as catalysts. J. Mater. Sci. 2020, 55, 6195–6241. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Wang, Y.-H. Nonlinear optical properties of metal nanoparticles: A review. RSC Adv. 2017, 7, 45129–45144. [Google Scholar] [CrossRef] [Green Version]
- Fantoni, A.; Fernandes, M.; Vygranenko, Y.; Vieira, M.; Oliveira-Silva, R.; Prazeres, D.M.; Ribeiro, A.P.; Alegria, E.C.B. Optical properties of metal nanoparticles embedded in amorphous silicon analysed using discrete dipole approximation. Proc. SPIE 2018, 10526, 1052609. [Google Scholar] [CrossRef]
- Barui, A.K.; Das, S.; Patra, C.R. 10—Biomedical applications of green-synthesized metal nanoparticles using polysaccharides. In Functional Polysaccharides for Biomedical Applications; Maiti, S., Jana, S., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 329–355. [Google Scholar]
- Khursheed, R.; Dua, K.; Vishwas, S.; Gulati, M.; Jha, N.K.; Aldhafeeri, G.M.; Alanazi, F.G.; Goh, B.H.; Gupta, G.; Paudel, K.R.; et al. Biomedical applications of metallic nanoparticles in cancer: Current status and future perspectives. Biomed. Pharmacother. 2022, 150, 112951. [Google Scholar] [CrossRef]
- Illath, K.; Wankhar, S.; Mohan, L.; Nagai, M.; Santra, T.S. Metallic Nanoparticles for Biomedical Applications. In Nanomaterials and Their Biomedical Applications; Santra, T.S., Mohan, L., Eds.; Springer: Singapore, 2021; pp. 29–81. [Google Scholar]
- Li, H.; Qiao, R.; Davis, T.P.; Tang, S.Y. Biomedical Applications of Liquid Metal Nanoparticles: A Critical Review. Biosensors 2020, 10, 196. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Mohamad Ibrahim, M.N. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef]
- Yougbare, S.; Chang, T.-K.; Tan, S.-H.; Kuo, J.-C.; Hsu, P.-H.; Su, C.-Y.; Kuo, T.-R. Antimicrobial gold nanoclusters: Recent developments and future perspectives. Int. J. Mol. Sci. 2019, 20, 2924. [Google Scholar] [CrossRef] [Green Version]
- Geng, H.; Vilms Pedersen, S.; Ma, Y.; Haghighi, T.; Dai, H.; Howes, P.D.; Stevens, M.M. Noble Metal Nanoparticle Biosensors: From Fundamental Studies toward Point-of-Care Diagnostics. Acc. Chem. Res. 2022, 55, 593–604. [Google Scholar] [CrossRef]
- Malathi, S.; Pakrudheen, I.; Kalkura, S.N.; Webster, T.J.; Balasubramanian, S. Disposable biosensors based on metal nanoparticles. Sens. Int. 2022, 3, 100169. [Google Scholar] [CrossRef]
- Yaraki, M.T.; Tan, Y.N. Metal Nanoparticles-Enhanced Biosensors: Synthesis, Design and Applications in Fluorescence Enhancement and Surface-enhanced Raman Scattering. Chem. Asian J. 2020, 15, 3180–3208. [Google Scholar] [CrossRef]
- Choi, H.K.; Lee, M.-J.; Lee, S.N.; Kim, T.-H.; Oh, B.-K. Noble Metal Nanomaterial-Based Biosensors for Electrochemical and Optical Detection of Viruses Causing Respiratory Illnesses. Front. Chem. 2021, 9, 672739. [Google Scholar] [CrossRef]
- Białas, K.; Moschou, D.; Marken, F.; Estrela, P. Electrochemical sensors based on metal nanoparticles with biocatalytic activity. Microchim. Acta 2022, 189, 172. [Google Scholar] [CrossRef]
- Wang, Y.; Fugetsu, B.; Sakata, I.; Fujisue, C.; Kabayama, S.; Tahara, N.; Morisawa, S. Monolayered Platinum Nanoparticles as Efficient Electrocatalysts for the Mass Production of Electrolyzed Hydrogen Water. Sci. Rep. 2020, 10, 10126. [Google Scholar] [CrossRef]
- Cheng, Y.; Fan, M.; Lin, W.; Zhang, Z.; Zhang, H. Platinum nanoparticles on defect-rich nitrogen-doped hollow carbon as an efficient electrocatalyst for hydrogen evolution reactions. RSC Adv. 2020, 10, 930–937. [Google Scholar] [CrossRef] [Green Version]
- Marinoiu, A.; Carcadea, E.; Sacca, A.; Carbone, A.; Sisu, C.; Dogaru, A.; Raceanu, M.; Varlam, M. One-step synthesis of graphene supported platinum nanoparticles as electrocatalyst for PEM fuel cells. Int. J. Hydrogen Energy 2021, 46, 12242–12253. [Google Scholar] [CrossRef]
- Razavi, M.; Sookhakian, M.; Goh, B.T.; Bahron, H.; Mahmoud, E.; Alias, Y. Molybdenum Disulfide Nanosheets Decorated with Platinum Nanoparticle as a High Active Electrocatalyst in Hydrogen Evolution Reaction. Nanoscale Res. Lett. 2022, 17, 9. [Google Scholar] [CrossRef]
- Can-Uc, B.; Rangel-Rojo, R.; Peña-Ramírez, A.; de Araújo, C.B.; Baltar, H.T.M.C.M.; Crespo-Sosa, A.; Garcia-Betancourt, M.L.; Oliver, A. Nonlinear optical response of platinum nanoparticles and platinum ions embedded in sapphire. Opt. Express 2016, 24, 9955–9965. [Google Scholar] [CrossRef]
- Ganeev, R.A.; Tugushev, R.I.; Usmanov, T. Application of the nonlinear optical properties of platinum nanoparticles for the mode locking of Nd:glass laser. Appl. Phys. B 2009, 94, 647–651. [Google Scholar] [CrossRef]
- Shen, Y.; Pan, T.; Wu, P.; Huang, J.; Li, H.; Khalil, I.E.; Li, S.; Zheng, B.; Wu, J.; Wang, Q.; et al. Regulating Electronic Status of Platinum Nanoparticles by Metal–Organic Frameworks for Selective Catalysis. CCS Chem. 2021, 3, 1607–1614. [Google Scholar] [CrossRef]
- Volkov, I.A.; Simonenko, N.P.; Efimov, A.A.; Simonenko, T.L.; Vlasov, I.S.; Borisov, V.I.; Arsenov, P.V.; Lebedinskii, Y.Y.; Markeev, A.M.; Lizunova, A.A.; et al. Platinum Based Nanoparticles Produced by a Pulsed Spark Discharge as a Promising Material for Gas Sensors. Appl. Sci. 2021, 11, 526. [Google Scholar] [CrossRef]
- Chen, J.; Lu, Y.; Yan, F.; Wu, Y.; Huang, D.; Weng, Z. A fluorescent biosensor based on catalytic activity of platinum nanoparticles for freshness evaluation of aquatic products. Food Chem. 2020, 310, 125922. [Google Scholar] [CrossRef]
- Özbek, M.A.; Yaşar, A.; Çete, S.; Er, E.; Erk, N. A novel biosensor based on graphene/platinum nanoparticles/Nafion composites for determination of glucose. J. Solid State Electrochem. 2021, 25, 1601–1610. [Google Scholar] [CrossRef]
- Liu, J.; Fan, Y.; Chen, G.; Liu, Y. Highly sensitive glutamate biosensor based on platinum nanoparticles decorated MXene-Ti3C2Tx for l-glutamate determination in foodstuffs. LWT 2021, 148, 111748. [Google Scholar] [CrossRef]
- Huynh, G.T.; Kesarwani, V.; Walker, J.A.; Frith, J.E.; Meagher, L.; Corrie, S.R. Review: Nanomaterials for Reactive Oxygen Species Detection and Monitoring in Biological Environments. Front. Chem. 2021, 9, 728717. [Google Scholar] [CrossRef]
- Duanghathaipornsuk, S.; Farrell, E.J.; Alba-Rubio, A.C.; Zelenay, P.; Kim, D.-S. Detection Technologies for Reactive Oxygen Species: Fluorescence and Electrochemical Methods and Their Applications. Biosensors 2021, 11, 30. [Google Scholar] [CrossRef]
- Dong, X.-Y.; Gao, Z.-W.; Yang, K.-F.; Zhang, W.-Q.; Xu, L.-W. Nanosilver as a new generation of silver catalysts in organic transformations for efficient synthesis of fine chemicals. Catal. Sci. Technol. 2015, 5, 2554–2574. [Google Scholar] [CrossRef]
- Shaker Ardakani, L.; Surendar, A.; Thangavelu, L.; Mandal, T. Silver nanoparticles (Ag NPs) as catalyst in chemical reactions. Synth. Commun. 2021, 51, 1516–1536. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Kuwahara, Y.; Mori, K.; Kamegawa, T.; Yamashita, H. Enhanced Catalysis of Plasmonic Silver Nanoparticles by a Combination of Macro-/Mesoporous Nanostructured Silica Support. J. Phys. Chem. C 2021, 125, 9150–9157. [Google Scholar] [CrossRef]
- Jouyban, A.; Rahimpour, E. Optical sensors based on silver nanoparticles for determination of pharmaceuticals: An overview of advances in the last decade. Talanta 2020, 217, 121071. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Papponen, P.; Johansson, A.; Puttaraksa, N.; Gilbert, L. Preparation of graphene nanocomposites from aqueous silver nitrate using graphene oxide’s peroxidase-like and carbocatalytic properties. Sci. Rep. 2020, 10, 5126. [Google Scholar] [CrossRef] [Green Version]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The Potential of Silver Nanoparticles for Antiviral and Antibacterial Applications: A Mechanism of Action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef]
- Urnukhsaikhan, E.; Bold, B.-E.; Gunbileg, A.; Sukhbaatar, N.; Mishig-Ochir, T. Antibacterial activity and characteristics of silver nanoparticles biosynthesized from Carduus crispus. Sci. Rep. 2021, 11, 21047. [Google Scholar] [CrossRef]
- Anees Ahmad, S.; Sachi Das, S.; Khatoon, A.; Tahir Ansari, M.; Afzal, M.; Saquib Hasnain, M.; Kumar Nayak, A. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [Green Version]
- Arif, R.; Uddin, R. A review on recent developments in the biosynthesis of silver nanoparticles and its biomedical applications. Med. Devices Sens. 2021, 4, e10158. [Google Scholar] [CrossRef]
- Almatroudi, A. Silver nanoparticles: Synthesis, characterisation and biomedical applications. Open Life Sci. 2020, 15, 819–839. [Google Scholar] [CrossRef]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of silver nanoparticles with different shapes. Arab. J. Chem. 2019, 12, 1823–1838. [Google Scholar] [CrossRef] [Green Version]
- Patil, R.S.; Kokate, M.R.; Salvi, P.P.; Kolekar, S.S. A novel one step synthesis of silver nanoparticles using room temperature ionic liquid and their biocidal activity. Comptes Rendus Chim. 2011, 14, 1122–1127. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, W.; Zhang, Q.; Li, H.; Zhang, F. In Situ One-Step Synthesis of Platinum Nanoparticles Supported on Metal–Organic Frameworks as an Effective and Stable Catalyst for Selective Hydrogenation of 5-Hydroxymethylfurfural. ACS Omega 2020, 5, 16183–16188. [Google Scholar] [CrossRef]
- Borowik, A.; Banasiuk, R.; Derewonko, N.; Rychlowski, M.; Krychowiak-Masnicka, M.; Wyrzykowski, D.; Ziabka, M.; Woziwodzka, A.; Krolicka, A.; Piosik, J. Interactions of newly synthesized platinum nanoparticles with ICR-191 and their potential application. Sci. Rep. 2019, 9, 4987. [Google Scholar] [CrossRef]
- Kinhal, K.V.; Sinha, S.; Ravisankar, A.; Bhatt, N.P.; Pushpavanam, S. Simultaneous Synthesis and Separation of Nanoparticles Using Aqueous Two-Phase Systems. ACS Sustain. Chem. Eng. 2020, 8, 3013–3025. [Google Scholar] [CrossRef]
- Wang, L.; Xia, C.; Lin, F.; Qin, H.; Liu, W.; Xie, D.; Pan, X.; Lu, Z.; Li, S.; Zhang, X. Two-step process for synthesizing wheat-like silver nanoparticles by using facile wet-chemical method. Mater. Res. Express 2019, 6, 115092. [Google Scholar] [CrossRef]
- LaGrow, A.P.; Knudsen, K.R.; AlYami, N.M.; Anjum, D.H.; Bakr, O.M. Effect of Precursor Ligands and Oxidation State in the Synthesis of Bimetallic Nano-Alloys. Chem. Mater. 2015, 27, 4134–4141. [Google Scholar] [CrossRef] [Green Version]
- Schütte, K.; Doddi, A.; Kroll, C.; Meyer, H.; Wiktor, C.; Gemel, C.; van Tendeloo, G.; Fischer, R.A.; Janiak, C. Colloidal nickel/gallium nanoalloys obtained from organometallic precursors in conventional organic solvents and in ionic liquids: Noble-metal-free alkyne semihydrogenation catalysts. Nanoscale 2014, 6, 5532–5544. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Koshiyama, T.; Watanabe, T.; Miyahara, M.T. Room-Temperature Synthesis of Ni and Pt-Co Alloy Nanoparticles Using a Microreactor. Front. Chem. Eng. 2021, 3, 780384. [Google Scholar] [CrossRef]
- Kulbakov, A.A.; Allix, M.; Rakhmatullin, A.; Mikheykin, A.S.; Popov, Y.V.; Smirnova, N.V.; Maslova, O.; Leontyev, I.N. In Situ Investigation of Non-Isothermal Decomposition of Pt Acetylacetonate as One-Step Size-Controlled Synthesis of Pt Nanoparticles. Phys. Status Solidi 2018, 215, 1800488. [Google Scholar] [CrossRef]
- Yin, X.; Shi, M.; Wu, J.; Pan, Y.-T.; Gray, D.L.; Bertke, J.A.; Yang, H. Quantitative Analysis of Different Formation Modes of Platinum Nanocrystals Controlled by Ligand Chemistry. Nano Lett. 2017, 17, 6146–6150. [Google Scholar] [CrossRef]
- Gong, X.; Yang, Y.; Zhang, L.; Zou, C.; Cai, P.; Chen, G.; Huang, S. Controlled synthesis of Pt nanoparticles via seeding growth and their shape-dependent catalytic activity. J. Colloid Interface Sci. 2010, 352, 379–385. [Google Scholar] [CrossRef]
- Křenek, T.; Kovářík, T.; Pola, M.; Jakubec, I.; Bezdička, P.; Bastl, Z.; Pokorná, D.; Urbanová, M.; Galíková, A.; Pola, J. Enhancement of thermal stability of silver(I) acetylacetonate by platinum(II) acetylacetonate. Thermochim. Acta 2013, 554, 1–7. [Google Scholar] [CrossRef]
- Vanegas, J.P.; Scaiano, J.C.; Lanterna, A.E. Thiol-Stabilized Gold Nanoparticles: New Ways To Displace Thiol Layers Using Yttrium or Lanthanide Chlorides. Langmuir 2017, 33, 12149–12154. [Google Scholar] [CrossRef]
- Battocchio, C.; Porcaro, F.; Mukherjee, S.; Magnano, E.; Nappini, S.; Fratoddi, I.; Quintiliani, M.; Russo, M.V.; Polzonetti, G. Gold Nanoparticles Stabilized with Aromatic Thiols: Interaction at the Molecule–Metal Interface and Ligand Arrangement in the Molecular Shell Investigated by SR-XPS and NEXAFS. J. Phys. Chem. C 2014, 118, 8159–8168. [Google Scholar] [CrossRef]
- Aktara, M.N.; Nayim, S.; Sahoo, N.K.; Hossain, M. The synthesis of thiol-stabilized silver nanoparticles and their application towards the nanomolar-level colorimetric recognition of glutathione. N. J. Chem. 2019, 43, 13480–13490. [Google Scholar] [CrossRef]
- Dong, H.; Dai, Y.; Zhang, X.; Zhang, Z.; Fu, S.; Zhong, Z. The influence of amine structures on the stability and catalytic activity of gold nanoparticles stabilized by amine-modified hyperbranched polymers. Nanotechnology 2018, 29, 055705. [Google Scholar] [CrossRef]
- Odrozek, K.; Maresz, K.; Koreniuk, A.; Prusik, K.; Mrowiec-Białoń, J. Amine-stabilized small gold nanoparticles supported on AlSBA-15 as effective catalysts for aerobic glucose oxidation. Appl. Catal. A Gen. 2014, 475, 203–210. [Google Scholar] [CrossRef]
- Mbanga, O.; Cukrowska, E.; Gulumian, M. Dissolution of citrate-stabilized, polyethylene glycol–coated carboxyl and amine-functionalized gold nanoparticles in simulated biological fluids and environmental media. J. Nanoparticle Res. 2021, 23, 29. [Google Scholar] [CrossRef]
- Corbierre, M.K.; Cameron, N.S.; Sutton, M.; Mochrie, S.G.J.; Lurio, L.B.; Rühm, A.; Lennox, R.B. Polymer-Stabilized Gold Nanoparticles and Their Incorporation into Polymer Matrices. J. Am. Chem. Soc. 2001, 123, 10411–10412. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.; Siwal, S.; Ul Islam, R.; Witcomb, M.J.; Mallick, K. Polymer stabilized silver nanoparticle: An efficient catalyst for proton-coupled electron transfer reaction and the electrochemical recognition of biomolecule. Chem. Phys. Lett. 2014, 608, 145–151. [Google Scholar] [CrossRef]
- Wilkins, L.E.; Hasan, M.; Fayter, A.E.R.; Biggs, C.; Walker, M.; Gibson, M.I. Site-specific conjugation of antifreeze proteins onto polymer-stabilized nanoparticles. Polym. Chem. 2019, 10, 2986–2990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.W.; Choi, S.-R.; Heo, J.H. Simultaneous Stabilization and Functionalization of Gold Nanoparticles via Biomolecule Conjugation: Progress and Perspectives. ACS Appl. Mater. Interfaces 2021, 13, 42311–42328. [Google Scholar] [CrossRef]
- Bhatia, S.; Camacho, L.C.; Haag, R. Pathogen Inhibition by Multivalent Ligand Architectures. J. Am. Chem. Soc. 2016, 138, 8654–8666. [Google Scholar] [CrossRef]
- Petitjean, S.J.L.; Chen, W.; Koehler, M.; Jimmidi, R.; Yang, J.; Mohammed, D.; Juniku, B.; Stanifer, M.L.; Boulant, S.; Vincent, S.P.; et al. Multivalent 9-O-Acetylated-sialic acid glycoclusters as potent inhibitors for SARS-CoV-2 infection. Nat. Commun. 2022, 13, 2564. [Google Scholar] [CrossRef]
- Makhani, E.Y.; Zhang, A.; Haun, J.B. Quantifying and controlling bond multivalency for advanced nanoparticle targeting to cells. Nano Converg. 2021, 8, 38. [Google Scholar] [CrossRef]
- Perumal, S.; Hofmann, A.; Scholz, N.; Rühl, E.; Graf, C. Kinetics Study of the Binding of Multivalent Ligands on Size-Selected Gold Nanoparticles. Langmuir 2011, 27, 4456–4464. [Google Scholar] [CrossRef]
- Skarżewski, J.; Daniluk, E. Lipophilic complexones, part 3. Synthesis of polyamines derived from 2-alkyl-1,3-propanediols and 2,2-bis(hydroxymethyl)alkanols. Mon. Chem. Chem. Mon. 1983, 114, 1071–1077. [Google Scholar] [CrossRef]
- Singh, S.; Bharti, A.; Meena, V. Green synthesis of multi-shaped silver nanoparticles: Optical, morphological and antibacterial properties. J. Mater. Sci. Mater. Electron. 2015, 26, 3638–3648. [Google Scholar] [CrossRef]
- Peng, S.; McMahon, J.M.; Schatz, G.C.; Gray, S.K.; Sun, Y. Reversing the size-dependence of surface plasmon resonances. Proc. Natl. Acad. Sci. USA 2010, 107, 14530–14534. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-S.; El-Sayed, M.A. Gold and Silver Nanoparticles in Sensing and Imaging: Sensitivity of Plasmon Response to Size, Shape, and Metal Composition. J. Phys. Chem. B 2006, 110, 19220–19225. [Google Scholar] [CrossRef]
- Smetana, A.B.; Klabunde, K.J.; Sorensen, C.M. Synthesis of spherical silver nanoparticles by digestive ripening, stabilization with various agents, and their 3-D and 2-D superlattice formation. J. Colloid Interface Sci. 2005, 284, 521–526. [Google Scholar] [CrossRef]
- Shimpi, J.R.; Sidhaye, D.S.; Prasad, B.L.V. Digestive Ripening: A Fine Chemical Machining Process on the Nanoscale. Langmuir 2017, 33, 9491–9507. [Google Scholar] [CrossRef] [Green Version]
- Rohse, P.; Weickert, S.; Drescher, M.; Wittmann, V. Precipitation-free high-affinity multivalent binding by inline lectin ligands. Chem. Sci. 2020, 11, 5227–5237. [Google Scholar] [CrossRef]
- Csizmar, C.M.; Petersburg, J.R.; Perry, T.J.; Rozumalski, L.; Hackel, B.J.; Wagner, C.R. Multivalent Ligand Binding to Cell Membrane Antigens: Defining the Interplay of Affinity, Valency, and Expression Density. J. Am. Chem. Soc. 2019, 141, 251–261. [Google Scholar] [CrossRef]
- Bakshi, A.K.; Haider, T.; Tiwari, R.; Soni, V. Critical parameters for design and development of multivalent nanoconstructs: Recent trends. Drug Deliv. Transl. Res. 2022, 11, 1–24. [Google Scholar] [CrossRef]
- Quinson, J.; Jensen, K.M.Ø. From platinum atoms in molecules to colloidal nanoparticles: A review on reduction, nucleation and growth mechanisms. Adv. Colloid Interface Sci. 2020, 286, 102300. [Google Scholar] [CrossRef]
- Moraes, L.C.; Figueiredo, R.C.; Espinós, J.P.; Vattier, F.; Franconetti, A.; Jaime, C.; Lacroix, B.; Rojo, J.; Lara, P.; Conejero, S. Platinum nanoparticles stabilized by N-heterocyclic thiones. Synthesis and catalytic activity in mono- and di-hydroboration of alkynes. Nanoscale 2020, 12, 6821–6831. [Google Scholar] [CrossRef]
- Axet, M.R.; Philippot, K.; Chaudret, B.; Cabié, M.; Giorgio, S.; Henry, C.R. TEM and HRTEM evidence for the role of ligands in the formation of shape-controlled platinum nanoparticles. Small 2011, 7, 235–241. [Google Scholar] [CrossRef]
- Michel, J.A.; Morris Iii, W.H.; Lukehart, C.M. Synthesis of shaped Pt nanoparticles using common anions or small molecules as shape-directing agents: Observation of a strong halide or pseudo-halide effect. J. Mater. Chem. A 2015, 3, 2012–2018. [Google Scholar] [CrossRef] [Green Version]
- Leong, G.J.; Schulze, M.C.; Strand, M.B.; Maloney, D.; Frisco, S.L.; Dinh, H.N.; Pivovar, B.; Richards, R.M. Shape-directed platinum nanoparticle synthesis: Nanoscale design of novel catalysts. Appl. Organomet. Chem. 2014, 28, 1–17. [Google Scholar] [CrossRef]
- Kulbakov, A.A.; Kuriganova, A.B.; Allix, M.; Rakhmatullin, A.; Smirnova, N.; Maslova, O.A.; Leontyev, I.N. Non-isothermal decomposition of platinum acetylacetonate as a cost-efficient and Size-Controlled Synthesis of Pt/C nanoparticles. Catal. Commun. 2018, 117, 14–18. [Google Scholar] [CrossRef]








| Temperature (°C) | Maximum (nm) | ||
|---|---|---|---|
| M-AgNPs | D-AgNPs | T-AgNPs | |
| 80 | 417 ± 1 | 415 ± 1 | 423 ± 1 |
| 90 | 417 ± 1 | 415 ± 1 | 421 ± 1 |
| 100 | 416 ± 1 | 414 ± 1 | 418 ± 1 |
| 110 | 415 ± 1 | 414 ± 1 | 414 ± 1 |
| 120 | 415 ± 1 | 414 ± 1 | 411 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perumal, S.; Atchudan, R.; Rühl, E.; Graf, C. Controlled Synthesis of Platinum and Silver Nanoparticles Using Multivalent Ligands. Nanomaterials 2022, 12, 2294. https://doi.org/10.3390/nano12132294
Perumal S, Atchudan R, Rühl E, Graf C. Controlled Synthesis of Platinum and Silver Nanoparticles Using Multivalent Ligands. Nanomaterials. 2022; 12(13):2294. https://doi.org/10.3390/nano12132294
Chicago/Turabian StylePerumal, Suguna, Raji Atchudan, Eckart Rühl, and Christina Graf. 2022. "Controlled Synthesis of Platinum and Silver Nanoparticles Using Multivalent Ligands" Nanomaterials 12, no. 13: 2294. https://doi.org/10.3390/nano12132294