Orientation-Mediated Luminescence Enhancement and Spin-Orbit Coupling in ZnO Single Crystals
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. Surface Morphology, X-ray Diffraction, and Raman Analysis
3.2. X-ray Photoelectron Spectroscopy Measurement
3.3. Atomic Force Microscopy Analysis
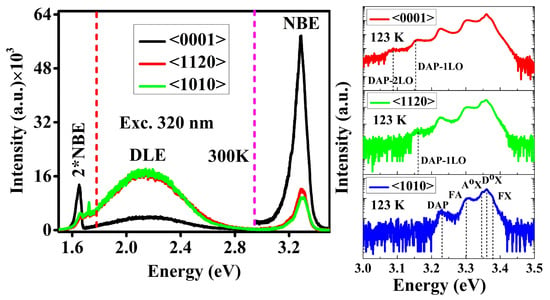
3.4. Room-Temperature and Low-Temperature Photoluminescence Spectroscopy
3.5. Excitation-Wavelength-Dependent Photoluminescence Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Dong, J.; Hesketh, P.J.; Liu, M. Synthesis and gas sensing properties of ZnO single crystal flakes. J. Mater. Chem. 2005, 15, 2316–2320. [Google Scholar] [CrossRef]
- Wu, T.; Deng, G.; Zhen, C. Metal oxide mesocrystals and mesoporous single crystals: Synthesis, properties and applications in solar energy conversion. J. Mater. Sci. Technol. 2021, 73, 9–22. [Google Scholar] [CrossRef]
- Dworschak, D.; Brunnhofer, C.; Valtiner, M. Photocorrosion of ZnO Single Crystals during Electrochemical Water Splitting. ACS Appl. Mater. Interfaces 2020, 12, 51530–51536. [Google Scholar] [CrossRef] [PubMed]
- Tishkevich, D.I.; Grabchikov, S.S.; Tsybulskaya, L.S.; Shendyukov, V.S.; Perevoznikov, S.S.; Trukhanov, S.V.; Trukhanova, E.L.; Trukhanov, A.V.; Vinnik, D.A. Electrochemical deposition regimes and critical influence of organic additives on the structure of Bi films. J. Alloys Compd. 2018, 735, 1943–1948. [Google Scholar] [CrossRef]
- Mukhanov, V.A.; Sokolov, P.S.; Baranov, A.N.; Timoshenko, V.Y.; Zhigunov, D.M.; Solozhenko, V.L. Congruent melting and rapid single-crystal growth of ZnO at 4 GPa. CrystEngComm 2013, 15, 6318–6322. [Google Scholar] [CrossRef]
- Lin, W.; Ding, K.; Lin, Z.; Zhang, J.; Huang, J.; Huang, F. The growth and investigation on Ga-doped ZnO single crystals with high thermal stability and high carrier mobility. CrystEngComm 2011, 13, 3338–3341. [Google Scholar] [CrossRef]
- Tishkevich, D.I.; Vorobjova, A.I.; Vinnik, D. Formation and Corrosion Behavior of Nickel/Alumina Nanocomposites. Solid State Phenom. 2020, 299, 100–106. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, M.; Sun, Y.; Zhang, Z.; Li, B.; Zhao, H.; Shan, C.; Shen, D. Electrically pumped Fabry–Perot microlasers from single Ga-doped ZnO microbelt based heterostructure diodes. Nanoscale 2018, 10, 18774–18785. [Google Scholar] [CrossRef]
- Liu, W.Z.; Xu, H.Y.; Wang, C.L.; Zhang, L.X.; Zhang, C.; Sun, S.Y.; Ma, J.G.; Zhang, X.T.; Wang, J.N.; Liu, Y.C. Enhanced ultraviolet emission and improved spatial distribution uniformity of ZnO nanorod array light-emitting diodes via Ag nanoparticles decoration. Nanoscale 2013, 5, 8634–8639. [Google Scholar] [CrossRef]
- Chen, D.; Xin, Y.; Lu, B.; Pan, X.; Huang, J.; He, H.; Ye, Z. Self-powered ultraviolet photovoltaic photodetector based on graphene/ZnO heterostructure. Appl. Surf. Sci. 2020, 529, 147087. [Google Scholar] [CrossRef]
- Nicholas, N.J.; Ducker, W.; Franks, G.V. Differential Etching of ZnO Native Planes under Basic Conditions. Langmuir 2012, 28, 5633–5641. [Google Scholar] [CrossRef] [PubMed]
- Yatskiv, R.; Grym, J.; Tiagulskyi, S.; Bašinová, N. Influence of Surface Polarity on Optoelectronic Properties of PEDOT:PSS/ZnO Hybrid Heterojunctions. Phys. Status Solidi (a) 2021, 218, 2000612. [Google Scholar] [CrossRef]
- Sasaki, H.; Kato, H.; Izumida, F.; Endo, H.; Maeda, K.; Ikeda, M.; Kashiwaba, Y.; Niikura, I.; Kashiwaba, Y. Photoluminescence properties of ZnO single crystals with polar and non-polar faces. Phys. Status Solidi (c) 2006, 3, 1034–1037. [Google Scholar] [CrossRef]
- Meyer, B.K.; Alves, H.; Hofmann, D.M.; Kriegseis, W.; Forster, D.; Bertram, F.; Christen, J.; Hoffmann, A.; Straßburg, M.; Dworzak, M.; et al. Bound exciton and donor–acceptor pair recombinations in ZnO. Phys. Status Solidi (b) 2004, 241, 231–260. [Google Scholar] [CrossRef]
- Ohashi, N.; Ishigaki, T.; Okada, N.; Taguchi, H.; Sakaguchi, I.; Hishita, S.; Sekiguchi, T.; Haneda, H. Passivation of active recombination centers in ZnO by hydrogen doping. J. Appl. Phys. 2003, 93, 6386–6392. [Google Scholar] [CrossRef]
- Yang, L.L.; Zhao, Q.X.; Willander, M.; Liu, X.J.; Fahlman, M.; Yang, J.H. Origin of the surface recombination centers in ZnO nanorods arrays by X-ray photoelectron spectroscopy. Appl. Surf. Sci. 2010, 256, 3592–3597. [Google Scholar] [CrossRef] [Green Version]
- Son, N.T.; Ivanov, I.G.; Kuznetsov, A.; Svensson, B.G.; Zhao, Q.X.; Willander, M.; Morishita, N.; Ohshima, T.; Itoh, H.; Isoya, J.; et al. Recombination centers in as-grown and electron-irradiated ZnO substrates. J. Appl. Phys. 2007, 102, 093504. [Google Scholar] [CrossRef]
- Cole, J.J.; Wang, X.; Knuesel, R.J.; Jacobs, H.O. Integration of ZnO Microcrystals with Tailored Dimensions Forming Light Emitting Diodes and UV Photovoltaic Cells. Nano Lett. 2008, 8, 1477–1481. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, L. The influence of orientation on the photoluminescence behavior of ZnO thin films obtained by chemical solution deposition. Mater. Lett. 2009, 63, 301–303. [Google Scholar] [CrossRef]
- Marin, O.; Tirado, M.; Budini, N.; Mosquera, E.; Figueroa, C.; Comedi, D. Photoluminescence from c-axis oriented ZnO films synthesized by sol-gel with diethanolamine as chelating agent. Mater. Sci. Semicond. Process. 2016, 56, 59–65. [Google Scholar] [CrossRef]
- Kalyanaraman, S.; Vettumperumal, R.; Thangavel, R. Study of multiple phonon behavior in Li-doped ZnO thin films fabricated using the sol-gel spin-coating technique. J. Korean Phys. Soc. 2013, 62, 804–808. [Google Scholar] [CrossRef]
- Cuscó, R.; Alarcón-Lladó, E.; Ibáñez, J.; Artús, L.; Jiménez, J.; Wang, B.; Callahan, M.J. Temperature dependence of Raman scattering in ZnO. Phys. Rev. B 2007, 75, 165202. [Google Scholar] [CrossRef]
- Ashkenov, N.; Mbenkum, B.N.; Bundesmann, C.; Riede, V.; Lorenz, M.; Spemann, D.; Kaidashev, E.M.; Kasic, A.; Schubert, M.; Grundmann, M.; et al. Infrared dielectric functions and phonon modes of high-quality ZnO films. J. Appl. Phys. 2002, 93, 126–133. [Google Scholar] [CrossRef]
- Giri, P.K.; Bhattacharyya, S.; Singh, D.K.; Kesavamoorthy, R.; Panigrahi, B.K.; Nair, K.G.M. Correlation between microstructure and optical properties of ZnO nanoparticles synthesized by ball milling. J. Appl. Phys. 2007, 102, 093515. [Google Scholar] [CrossRef] [Green Version]
- Lima, L.; Caldas, L.D.S.; Alí, A.; Barreto, J.; Freitas, R.; Mazzarella, A.; Felix, G.; Carozo, V.; Stavale, F. Growth and Raman spectroscopy of ultrathin ZnO(0001) films on Ag(001). Surf. Sci. 2021, 704, 121748. [Google Scholar] [CrossRef]
- Calleja, J.M.; Cardona, M. Resonant Raman scattering in ZnO. Phys. Rev. B 1977, 16, 3753–3761. [Google Scholar] [CrossRef]
- Satyarthi, P.; Ghosh, S.; Mishra, P.; Sekhar, B.R.; Singh, F.; Kumar, P.; Kanjilal, D.; Dhaka, R.S.; Srivastava, P. Defect controlled ferromagnetism in xenon ion irradiated zinc oxide. J. Magn. Magn. Mater. 2015, 385, 318–325. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Radiman, S.; Daud, A.R.; Tabet, N.; Al-Douri, Y. XPS and optical studies of different morphologies of ZnO nanostructures prepared by microwave methods. Ceram. Int. 2013, 39, 2283–2292. [Google Scholar] [CrossRef]
- Tay, Y.Y.; Tan, T.T.; Liang, M.H.; Boey, F.; Li, S. Specific defects, surface band bending and characteristic green emissions of ZnO. Phys. Chem. Chem. Phys. 2010, 12, 6008–6013. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, X.; Yu, Y.H.; Pei, Z.L.; Bai, X.D.; Sun, C.; Huang, R.F.; Wen, L.S. X-ray photoelectron spectroscopy and auger electron spectroscopy studies of Al-doped ZnO films. Appl. Surf. Sci. 2000, 158, 134–140. [Google Scholar] [CrossRef]
- Pandey, B.; Ghosh, S.; Srivastava, P.; Kumar, P.; Kanjilal, D. Influence of microstructure on room temperature ferromagnetism in Ni implanted nanodimensional ZnO films. J. Appl. Phys. 2009, 105, 033909. [Google Scholar] [CrossRef]
- Hassan, A.; Jin, Y.; Irfan, M.; Jiang, Y. Acceptor-modulated optical enhancements and band-gap narrowing in ZnO thin films. AIP Adv. 2018, 8, 035212. [Google Scholar] [CrossRef]
- Fang, Z.B.; Yan, Z.J.; Tan, Y.S.; Liu, X.Q.; Wang, Y.Y. Influence of post-annealing treatment on the structure properties of ZnO films. Appl. Surf. Sci. 2005, 241, 303–308. [Google Scholar] [CrossRef]
- Clark, S.J.; Robertson, J.; Lany, S.; Zunger, A. Intrinsic defects in ZnO calculated by screened exchange and hybrid density functionals. Phys. Rev. B 2010, 81, 115311. [Google Scholar] [CrossRef] [Green Version]
- Vidya, R.; Ravindran, P.; Fjellvåg, H.; Svensson, B.G.; Monakhov, E.; Ganchenkova, M.; Nieminen, R.M. Energetics of intrinsic defects and their complexes in ZnO investigated by density functional calculations. Phys. Rev. B 2011, 83, 045206. [Google Scholar] [CrossRef] [Green Version]
- Cui, M.; Zhang, Z.; Wang, Y.; Finch, A.; Townsend, P.D. Temperature dependence of bulk luminescence from ZnO. Luminescence 2018, 33, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Janotti, A.; Van de Walle, C.G. Oxygen vacancies in ZnO. Appl. Phys. Lett. 2005, 87, 122102. [Google Scholar] [CrossRef]
- Lin, B.; Fu, Z.; Jia, Y. Green luminescent center in undoped zinc oxide films deposited on silicon substrates. Appl. Phys. Lett. 2001, 79, 943–945. [Google Scholar] [CrossRef]
- Lima, S.A.M.; Sigoli, F.A.; Jafelicci Jr, M.; Davolos, M.R. Luminescent properties and lattice defects correlation on zinc oxide. Int. J. Inorg. Mater. 2001, 3, 749–754. [Google Scholar] [CrossRef]
- Xu, P.S.; Sun, Y.M.; Shi, C.S.; Xu, F.Q.; Pan, H.B. The electronic structure and spectral properties of ZnO and its defects. Nucl. Instrum. Methods Phys. Res. Sect. B: Beam Interact. Mater. At. 2003, 199, 286–290. [Google Scholar] [CrossRef]
- Nickel, N.H.; Terukov, E. Zinc Oxide—A Material for Micro-and Optoelectronic Applications. In Proceedings of the NATO Advanced Research Workshop on Zinc Oxide as a Material for Micro-and Optoelectronic Applications, Saint Petersburg, Russia, 23–25 June 2004; Springer Science & Business Media: Dordrecht, The Netherland, 2006; Volume 194. [Google Scholar]
- Oba, F.; Togo, A.; Tanaka, I.; Paier, J.; Kresse, G. Defect energetics in ZnO: A hybrid Hartree-Fock density functional study. Phys. Rev. B 2008, 77, 245202. [Google Scholar] [CrossRef] [Green Version]
- Bjørheim, T.S.; Kotomin, E. Ab Initio Thermodynamics of Oxygen Vacancies and Zinc Interstitials in ZnO. J. Phys. Chem. Lett. 2014, 5, 4238–4242. [Google Scholar] [CrossRef] [PubMed]
- van Sark, W.G.J.H.M.; Frederix, P.L.T.M.; Bol, A.A.; Gerritsen, H.C.; Meijerink, A. Blueing, Bleaching, and Blinking of Single CdSe/ZnS Quantum Dots. ChemPhysChem 2002, 3, 871–879. [Google Scholar] [CrossRef] [Green Version]
- Derfus, A.M.; Chan, W.C.W.; Bhatia, S.N. Probing the Cytotoxicity of Semiconductor Quantum Dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Ozasa, K.; Nemoto, S.; Maeda, M.; Hara, M. Excitation-wavelength-dependent photoluminescence evolution of CdSe∕ZnS nanoparticles. J. Appl. Phys. 2007, 101, 103503. [Google Scholar] [CrossRef]
- Zhang, X.T.; Liu, Y.C.; Zhi, Z.Z.; Zhang, J.Y.; Lu, Y.M.; Shen, D.Z.; Xu, W.; Fan, X.W.; Kong, X.G. Temperature dependence of excitonic luminescence from nanocrystalline ZnO films. J. Lumin. 2002, 99, 149–154. [Google Scholar] [CrossRef]
- Li, T.; Zhu, Y.; Ji, X.; Zheng, W.; Lin, Z.; Lu, X.; Huang, F. Experimental Evidence on Stability of N Substitution for O in ZnO Lattice. J. Phys. Chem. Lett. 2020, 11, 8901–8907. [Google Scholar] [CrossRef]
- Huang, G.-Y.; Wang, C.-Y.; Wang, J.-T. First-principles study of diffusion of oxygen vacancies and interstitials in ZnO. J. Phys.: Condens. Matter 2009, 21, 195403. [Google Scholar] [CrossRef]
- Li, H.; Schirra, L.K.; Shim, J.; Cheun, H.; Kippelen, B.; Monti, O.L.A.; Bredas, J.-L. Zinc Oxide as a Model Transparent Conducting Oxide: A Theoretical and Experimental Study of the Impact of Hydroxylation, Vacancies, Interstitials, and Extrinsic Doping on the Electronic Properties of the Polar ZnO (0002) Surface. Chem. Mater. 2012, 24, 3044–3055. [Google Scholar] [CrossRef]
- Zhang, M.; Averseng, F.; Krafft, J.-M.; Borghetti, P.; Costentin, G.; Stankic, S. Controlled Formation of Native Defects in Ultrapure ZnO for the Assignment of Green Emissions to Oxygen Vacancies. J. Phys. Chem. C 2020, 124, 12696–12704. [Google Scholar] [CrossRef]
- Wu, K.; He, H.; Lu, Y.; Huang, J.; Ye, Z. Dominant free exciton emission in ZnO nanorods. Nanoscale 2012, 4, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Varshni, Y.P. Temperature dependence of the energy gap in semiconductors. Physica 1967, 34, 149–154. [Google Scholar] [CrossRef]
- Rai, R.C.; Guminiak, M.; Wilser, S.; Cai, B.; Nakarmi, M.L. Elevated temperature dependence of energy band gap of ZnO thin films grown by e-beam deposition. J. Appl. Phys. 2012, 111, 073511. [Google Scholar] [CrossRef]
- Wang, L.; Giles, N.C. Temperature dependence of the free-exciton transition energy in zinc oxide by photoluminescence excitation spectroscopy. J. Appl. Phys. 2003, 94, 973–978. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.S.; Ko, W.B.; Sohn, J.I.; Cha, S.N.; Kim, J.M.; Park, Y.J.; Hong, J.P. Photoluminescence Analysis of Energy Level on Li-Doped ZnO Nanowires Grown by a Hydrothermal Method. Appl. Phys. Express 2012, 5, 095002. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, B.; Sun, X.; Fu, Z. Temperature-dependent photoluminescence of nanocrystalline ZnO thin films grown on Si (100) substrates by the sol–gel process. Appl. Phys. Lett. 2005, 86, 131910. [Google Scholar] [CrossRef]
- Fonoberov, V.A.; Alim, K.A.; Balandin, A.A.; Xiu, F.; Liu, J. Photoluminescence investigation of the carrier recombination processes in ZnO quantum dots and nanocrystals. Phys. Rev. B 2006, 73, 165317. [Google Scholar] [CrossRef] [Green Version]
- Lambrecht, W.R.L.; Rodina, A.V.; Limpijumnong, S.; Segall, B.; Meyer, B.K. Valence-band ordering and magneto-optic exciton fine structure in ZnO. Phys. Rev. B 2002, 65, 075207. [Google Scholar] [CrossRef]
- Reynolds, D.C.; Look, D.C.; Jogai, B.; Litton, C.W.; Cantwell, G.; Harsch, W.C. Valence-band ordering in ZnO. Phys. Rev. B 1999, 60, 2340–2344. [Google Scholar] [CrossRef] [Green Version]
- Christopher, J.W.; Goldberg, B.B.; Swan, A.K. Long tailed trions in monolayer MoS2: Temperature dependent asymmetry and resulting red-shift of trion photoluminescence spectra. Sci. Rep. 2017, 7, 14062. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.B.; Thomas, M.A. Power dependent photoluminescence of ZnO. J. Appl. Phys. 2009, 106, 033518. [Google Scholar] [CrossRef]
- Schmidt, T.; Lischka, K.; Zulehner, W. Excitation-power dependence of the near-band-edge photoluminescence of semiconductors. Phys. Rev. B 1992, 45, 8989–8994. [Google Scholar] [CrossRef] [PubMed]
- Kurbanov, S.; Igamberdiev, K.; Yuldashev, S.; Kang, T.W. Excitation Intensity Dependent Studies of Photoluminescence from ZnO Nanocrystals Deposited on Different Substrates. Jpn. J. Appl. Phys. 2009, 48, 115004. [Google Scholar] [CrossRef]











| Sr# | Raman Shift (cm−1) of ZnO Single Crystals Measured in the Current Study | Raman Shift (cm−1) of ZnO Single Crystals Reported in the Literature [24,25] | Peak Identity | Symmetry |
|---|---|---|---|---|
| 1 | 97 | 99 | E2(low) | E2 (Brillouin zone Γ) |
| 2 | 331 | 333 | E2(high)−E2(low) | E2, E1 (Brillouin zone Γ) |
| 3 | 375 | 379 | A1(TO) | A1 (Brillouin zone Γ) |
| 4 | 435 | 438 | E2(high) | E2 (Brillouin zone Γ) |
| 5 | 518 | AM | - | |
| 6 | 1097 | 1102 | 2A1(LO) + E1(LO) | A1, E1 |
| 7 | 1136 | 1138 | A1, 2LO | - |
| 8 | 1159 | 1154 | 2LO | Wurtzite ZnO, 2LO |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, A.; Khan, A.A.; Ahn, Y.H.; Azam, M.; Zubair, M.; Xue, W.; Cao, Y. Orientation-Mediated Luminescence Enhancement and Spin-Orbit Coupling in ZnO Single Crystals. Nanomaterials 2022, 12, 2192. https://doi.org/10.3390/nano12132192
Hassan A, Khan AA, Ahn YH, Azam M, Zubair M, Xue W, Cao Y. Orientation-Mediated Luminescence Enhancement and Spin-Orbit Coupling in ZnO Single Crystals. Nanomaterials. 2022; 12(13):2192. https://doi.org/10.3390/nano12132192
Chicago/Turabian StyleHassan, Ali, Abbas Ahmad Khan, Yeong Hwan Ahn, Muhammad Azam, Muhammad Zubair, Wei Xue, and Yu Cao. 2022. "Orientation-Mediated Luminescence Enhancement and Spin-Orbit Coupling in ZnO Single Crystals" Nanomaterials 12, no. 13: 2192. https://doi.org/10.3390/nano12132192