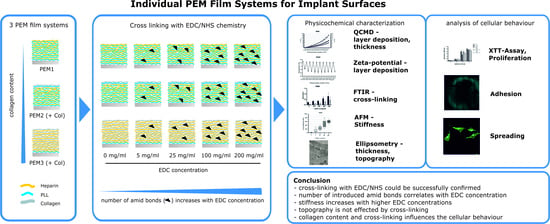
Tailored Polyelectrolyte Multilayer Systems by Variation of Polyelectrolyte Composition and EDC/NHS Cross-Linking: Physicochemical Characterization and In Vitro Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Multilayer Film Preparation and Cross-Linking
2.2. Films Characterization by Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D)
2.3. Cross-Linking Analysis by FTIR
2.4. AFM Measurements
2.5. Thickness and Topography Analysis with In Situ-Ellipsometry
2.6. Zeta Potential Measurements
2.7. Cell Culture Experiments
2.8. Statistical Analysis
3. Results
3.1. Film Construction and Kinetics
3.2. Film Properties after Cross-Linking
3.2.1. Confirmation of the EDC/NHS Cross-Linking with FTIR Measurements
3.2.2. Cross-Linking Changes Viscoelasticity
3.2.3. Thickness and Topography Analysis Performed by Ellipsometry
3.3. Cellular Response to EDC Cross-Linked PEMs
4. Discussion
4.1. Characteristics of PEM Films
4.2. Film Cross-Linking, Physiochemistry, and Topography of PEMs
4.3. Impact of Cross-Linking on Cellular Response
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Criado-Gonzalez, M.; Mijangos, C.; Hernandez, R. Polyelectrolyte Multilayer Films Based on Natural Polymers: From Fundamentals to Bio-Applications. Polymers 2021, 13, 2254. [Google Scholar] [CrossRef] [PubMed]
- Pahal, S.; Gakhar, R.; Raichur, A.M.; Varma, M.M. Polyelectrolyte multilayers for bio-applications: Recent advancements. IET Nanobiotechnol. 2017, 11, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Petrila, L.M.; Bucatariu, F.; Mihai, M.; Teodosiu, C. Polyelectrolyte Multilayers: An Overview on Fabrication, Properties, and Biomedical and Environmental Applications. Materials 2021, 14, 4152. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Han, F.; Zhao, P.; Lin, C.; Wen, X.; Ye, X. Layer-by-layer self-assembled multilayers on PEEK implants improve osseointegration in an osteoporosis rabbit model. Nanomedicine 2017, 13, 1423–1433. [Google Scholar] [CrossRef]
- Shi, Q.; Qian, Z.; Liu, D.; Liu, H. Surface Modification of Dental Titanium Implant by Layer-by-Layer Electrostatic Self-Assembly. Front. Physiol. 2017, 8, 574. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Luo, Z.; Hu, Y.; Shen, X.; Li, M.; Li, L.; Zhang, Y.; Yang, W.; Liu, P.; Cai, K. Enhancement of local bone remodeling in osteoporotic rabbits by biomimic multilayered structures on Ti6Al4V implants. J. Biomed. Mater. Res. A 2016, 104, 1437–1451. [Google Scholar] [CrossRef]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural Polymeric Scaffolds in Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef]
- Li, H.; Nie, B.; Zhang, S.; Long, T.; Yue, B. Immobilization of type I collagen/hyaluronic acid multilayer coating on enoxacin loaded titania nanotubes for improved osteogenesis and osseointegration in ovariectomized rats. Colloids Surf. B Biointerfaces 2019, 175, 409–420. [Google Scholar] [CrossRef]
- Bae, E.B.; Yoo, J.H.; Jeong, S.I.; Kim, M.S.; Lim, Y.M.; Ahn, J.J.; Lee, J.J.; Lee, S.H.; Kim, H.J.; Huh, J.B. Effect of Titanium Implants Coated with Radiation-Crosslinked Collagen on Stability and Osseointegration in Rat Tibia. Materials 2018, 11, 2520. [Google Scholar] [CrossRef] [Green Version]
- Cho, W.T.; Kim, S.Y.; Jung, S.I.; Kang, S.S.; Kim, S.E.; Hwang, S.H.; Jeong, C.M.; Huh, J.B. Effects of Gamma Radiation-Induced Crosslinking of Collagen Type I Coated Dental Titanium Implants on Osseointegration and Bone Regeneration. Materials 2021, 14, 3268. [Google Scholar] [CrossRef]
- Kellesarian, S.V.; Malignaggi, V.R.; Kellesarian, T.V.; Bashir Ahmed, H.; Javed, F. Does incorporating collagen and chondroitin sulfate matrix in implant surfaces enhance osseointegration? A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2018, 47, 241–251. [Google Scholar] [CrossRef]
- Hosoyama, K.; Lazurko, C.; Munoz, M.; McTiernan, C.D.; Alarcon, E.I. Peptide-Based Functional Biomaterials for Soft-Tissue Repair. Front. Bioeng. Biotechnol. 2019, 7, 205. [Google Scholar] [CrossRef] [PubMed]
- Morin, R.; Kaplan, D.; Perez-Ramirez, B. Bone Morphogenetic Protein-2 Binds as Multilayers to A Collagen Delivery Matrix: An Equilibrium Thermodynamic Analysis. Biomacromolecules 2006, 7, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O.; Naba, A. Overview of the matrisome--an inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 2012, 4, a004903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallet, S.D.; Clerc, O.; Ricard-Blum, S. Glycosaminoglycan-Protein Interactions: The First Draft of the Glycosaminoglycan Interactome. J. Histochem. Cytochem. 2021, 69, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Rider, C.C.; Mulloy, B. Heparin, Heparan Sulphate and the TGF-beta Cytokine Superfamily. Molecules 2017, 22, 713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hachim, D.; Whittaker, T.E.; Kim, H.; Stevens, M.M. Glycosaminoglycan-based biomaterials for growth factor and cytokine delivery: Making the right choices. J. Control. Release 2019, 313, 131–147. [Google Scholar] [CrossRef]
- Ren, K.; Crouzier, T.; Roy, C.; Picart, C. Polyelectrolyte multilayer films of controlled stiffness modulate myoblast cells differentiation. Adv. Funct. Mater. 2008, 18, 1378–1389. [Google Scholar] [CrossRef] [Green Version]
- Engler, A.J.; Richert, L.; Wong, J.Y.; Picart, C.; Discher, D.E. Surface probe measurements of the elasticity of sectioned tissue, thin gels and polyelectrolyte multilayer films: Correlations between substrate stiffness and cell adhesion. Surf. Sci. 2004, 570, 142–154. [Google Scholar] [CrossRef] [Green Version]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [Green Version]
- Mullen, C.A.; Vaughan, T.J.; Billiar, K.L.; McNamara, L.M. The effect of substrate stiffness, thickness, and cross-linking density on osteogenic cell behavior. Biophys. J. 2015, 108, 1604–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghiorghita, C.A.; Bucatariu, F.; Dragan, E.S. Influence of cross-linking in loading/release applications of polyelectrolyte multilayer assemblies. A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110050. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Huang, T.; Shi, F. Covalent layer-by-layer films: Chemistry, design, and multidisciplinary applications. Chem. Soc. Rev. 2018, 47, 5061–5098. [Google Scholar] [CrossRef] [PubMed]
- Francius, G.; Hemmerle, J.; Ohayon, J.; Schaaf, P.; Voegel, J.C.; Picart, C.; Senger, B. Effect of crosslinking on the elasticity of polyelectrolyte multilayer films measured by colloidal probe AFM. Microsc. Res. Tech. 2006, 69, 84–92. [Google Scholar] [CrossRef]
- Schneider, A.; Francius, G.; Obeid, R.; Schwinté, P.; Hemmerlé, J.; Frisch, B.; Schaaf, P.; Voegel, J.-C.; Senger, B.; Picart, C. Polyelectrolyte multilayers with a tunable Young’s modulus: Influence of film stiffness on cell adhesion. Langmuir 2006, 22, 1193–1200. [Google Scholar] [CrossRef]
- Mzyk, A.; Lackner, J.M.; Wilczek, P.; Lipińska, L.; Niemiec-Cyganek, A.; Samotus, A.; Morenc, M. Polyelectrolyte multilayer film modification for chemo-mechano-regulation of endothelial cell response. RSC Adv. 2016, 6, 8811–8828. [Google Scholar] [CrossRef]
- Niepel, M.S.; Almouhanna, F.; Ekambaram, B.K.; Menzel, M.; Heilmann, A.; Groth, T. Cross-linking multilayers of poly-l-lysine and hyaluronic acid: Effect on mesenchymal stem cell behavior. Int. J. Artifical Organs 2018, 41, 223–235. [Google Scholar] [CrossRef]
- Guillot, R.; Gilde, F.; Becquart, P.; Sailhan, F.; Lapeyrere, A.; Logeart-Avramoglou, D.; Picart, C. The stability of BMP loaded polyelectrolyte multilayer coatings on titanium. Biomaterials 2013, 34, 5737–5746. [Google Scholar] [CrossRef] [Green Version]
- Grohmann, S.; Rothe, H.; Frant, M.; Liefeith, K. Colloidal force spectroscopy and cell biological investigations on biomimetic polyelectrolyte multilayer coatings composed of chondroitin sulfate and heparin. Biomacromolecules 2011, 12, 1987–1997. [Google Scholar] [CrossRef]
- Picart, C. Molecular basis for the explanation of the exponential growth of polyelectrolyte multilayers. Proc. Natl. Acad. Sci. USA 2002, 99, 12531–12535. [Google Scholar] [CrossRef] [Green Version]
- Crouzier, T.; Picart, C. Ion pairing and hydration in polyelectrolyte multilayer films containing polysaccharides. Biomacromolecules 2009, 10, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Senger, B.; Vautier, D.; Picart, C.; Schaaf, P.; Voegel, J.C.; Lavalle, P. Natural polyelectrolyte films based on layer-by layer deposition of collagen and hyaluronic acid. Biomaterials 2005, 26, 3353–3361. [Google Scholar] [CrossRef] [PubMed]
- Richert, L.; Boulmedais, F.; Lavalle, P.; Mutterer, J.; Ferreux, E.; Decher, G.; Schaaf, P.; Voegel, J.C.; Picart, C. Improvement of stability and cell adhesion properties of polyelectrolyte multilayer films by chemical cross-linking. Biomacromolecules 2004, 5, 284–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamin, G.; Keselowsky, D.M.C.; García, A.J. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proc. Natl. Acad. Sci. USA 2005, 102, 5953–5957. [Google Scholar] [CrossRef] [Green Version]
- Khatiwala, C.B.; Peyton, S.R.; Putnam, A.J. Intrinsic mechanical properties of the extracellular matrix affect the behavior of pre-osteoblastic MC3T3-E1 cells. Am. J. Physiol. Cell Physiol. 2006, 290, C1640–C1650. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Hamaia, S.; Bax, D.V.; Malcor, J.D.; Schuster, C.F.; Gullberg, D.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Selecting the correct cellular model for assessing of the biological response of collagen-based biomaterials. Acta Biomater. 2018, 65, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Picart, C.; Senger, B.; Schaaf, P.; Voegel, J.C.; Frisch, B. Layer-by-layer films from hyaluronan and amine-modified hyaluronan. Langmuir 2007, 23, 2655–2662. [Google Scholar] [CrossRef] [Green Version]
- Richert, L.; Lavalle, P.; Payan, E.; Shu, X.Z.; Prestwich, G.D.; Stoltz, J.F.; Schaaf, P.; Voegel, J.C.; Picart, C. Layer by layer buildup of polysaccharide films: Physical chemistry and cellular adhesion aspects. Langmuir 2004, 20, 448–458. [Google Scholar] [CrossRef]
- Kovacevic, D.; Van der Burgh, S.; De Keizer, A.; Cohen Stuart, M.A. Kinetics of Formation and Dissolution of Weak Polyelectrolyte Multilayers: Role of Salt and Free Polyions. Langmuir 2002, 18, 5607–5612. [Google Scholar] [CrossRef]
- Tanchak, O.M.; Yager, K.G.; Fritzsche, H.; Harroun, T.; Katsaras, J.; Barrett, C.J. Water distribution in multilayers of weak polyelectrolytes. Langmuir 2006, 22, 5137–5143. [Google Scholar] [CrossRef]
- Barbani, N.; Lazzeri, L.; Cristallini, C.; Cascone, M.G.; Polacco, G.; Pizzirani, G. Bioartificial materials based on blends of collagen and poly(acrylic acid). J. Appl. Polym. Sci. 1999, 72, 971–976. [Google Scholar] [CrossRef]
- Johansson, J.A.; Halthur, T.; Herranen, M.; Soderberg, L.; Elofsson, U.; Hilborn, J. Build-up of collagen and hyaluronic acid polyelectrolyte multilayers. Biomacromolecules 2005, 6, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.D. Ion hydration: Implications for cellular function, polyelectrolytes, and protein crystallization. Biophys. Chem. 2006, 119, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Amorim, S.; Reis, C.A.; Reis, R.L.; Pires, R.A. Extracellular Matrix Mimics Using Hyaluronan-Based Biomaterials. Trends Biotechnol. 2021, 39, 90–104. [Google Scholar] [CrossRef]
- Soares da Costa, D.; Reis, R.L.; Pashkuleva, I. Sulfation of Glycosaminoglycans and Its Implications in Human Health and Disorders. Annu. Rev. Biomed. Eng. 2017, 19, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, D.V.; Shepherd, J.H.; Ghose, S.; Kew, S.J.; Cameron, R.E.; Best, S.M. The process of EDC-NHS Cross-linking of reconstituted collagen fibres increases collagen fibrillar order and alignment. APL Mater. 2015, 3, 014902. [Google Scholar] [CrossRef] [Green Version]
- Yang, C. Enhanced physicochemical properties of collagen by using EDC/NHS-crosslinking. Bull. Mater. Sci. 2012, 35, 913–918. [Google Scholar] [CrossRef]
- Usha, R.; Sreeram, K.J.; Rajaram, A. Stabilization of collagen with EDC/NHS in the presence of L-lysine: A comprehensive study. Colloids Surf. B Biointerfaces 2012, 90, 83–90. [Google Scholar] [CrossRef]
- Bax, D.V.; Davidenko, N.; Gullberg, D.; Hamaia, S.W.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Fundamental insight into the effect of carbodiimide crosslinking on cellular recognition of collagen-based scaffolds. Acta Biomater. 2017, 49, 218–234. [Google Scholar] [CrossRef]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Raynal, N.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 2015, 25, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Ao, H.; Xie, Y.; Tan, H.; Yang, S.; Li, K.; Wu, X.; Zheng, X.; Tang, T. Fabrication and in vitro evaluation of stable collagen/hyaluronic acid biomimetic multilayer on titanium coatings. J. R. Soc. Interface 2013, 10, 20130070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothe, H.; Rost, J.; Kramer, F.; Alkhatib, Y.; Petzold-Welcke, K.; Klemm, D.; Fischer, D.; Liefeith, K. Bacterial nanocellulose: Reinforcement of compressive strength using an adapted Mobile Matrix Reservoir Technology and suitable post-modification strategies, J. Mech. Behav. Biomed. Mater. 2022, 125, 104978. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Zhu, Y.; Liu, Y.; Ma, Z.; Gao, C.; Shen, J. Layer-by-layer assembly of chondroitin sulfate and collagen on aminolyzed poly(L-lactic acid) porous scaffolds to enhance their chondrogenesis. Acta Biomater. 2007, 3, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.L.; McDevitt, T.C.; Engler, A.J. Materials as stem cell regulators. Nat. Mater. 2014, 13, 547–557. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [Green Version]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of cell binding to collagen and gelatin: A study of the effect of 2D and 3D architecture and surface chemistry. J. Mater. Sci. Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef] [Green Version]
- Engler, A.J.; Griffin, M.A.; Sen, S.; Bonnemann, C.G.; Sweeney, H.L.; Discher, D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness: Pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004, 166, 877–887. [Google Scholar] [CrossRef] [Green Version]
- Grover, C.N.; Gwynne, J.H.; Pugh, N.; Hamaia, S.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Crosslinking and composition influence the surface properties, mechanical stiffness and cell reactivity of collagen-based films. Acta Biomater. 2012, 8, 3080–3090. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Abdeen, A.A.; Huang, T.H.; Kilian, K.A. Controlling cell geometry on substrates of variable stiffness can tune the degree of osteogenesis in human mesenchymal stem cells. J. Mech. Behav. Biomed. Mater. 2014, 38, 209–218. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schirmer, U.; Ludolph, J.; Rothe, H.; Hauptmann, N.; Behrens, C.; Bittrich, E.; Schliephake, H.; Liefeith, K. Tailored Polyelectrolyte Multilayer Systems by Variation of Polyelectrolyte Composition and EDC/NHS Cross-Linking: Physicochemical Characterization and In Vitro Evaluation. Nanomaterials 2022, 12, 2054. https://doi.org/10.3390/nano12122054
Schirmer U, Ludolph J, Rothe H, Hauptmann N, Behrens C, Bittrich E, Schliephake H, Liefeith K. Tailored Polyelectrolyte Multilayer Systems by Variation of Polyelectrolyte Composition and EDC/NHS Cross-Linking: Physicochemical Characterization and In Vitro Evaluation. Nanomaterials. 2022; 12(12):2054. https://doi.org/10.3390/nano12122054
Chicago/Turabian StyleSchirmer, Uwe, Johanna Ludolph, Holger Rothe, Nicole Hauptmann, Christina Behrens, Eva Bittrich, Henning Schliephake, and Klaus Liefeith. 2022. "Tailored Polyelectrolyte Multilayer Systems by Variation of Polyelectrolyte Composition and EDC/NHS Cross-Linking: Physicochemical Characterization and In Vitro Evaluation" Nanomaterials 12, no. 12: 2054. https://doi.org/10.3390/nano12122054