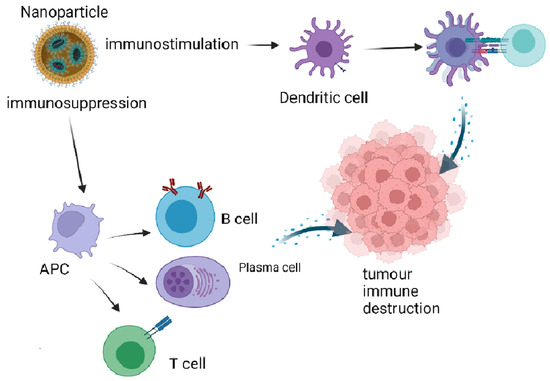
Moving Forward in Nano-Immune Interactions
Funding
Acknowledgments
Conflicts of Interest
References
- Inglut, C.T.; Sorrin, A.J.; Kuruppu, T.; Vig, S.; Cicalo, J.; Ahmad, H.; Huang, H.C. Immunological and Toxicological Considerations for the Design of Liposomes. Nanomaterials 2020, 10, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dukhinova, M.S.; Prilepskii, A.Y.; Shtil, A.A.; Vinogradov, V.V. Metal Oxide Nanoparticles in Therapeutic Regulation of Macrophage Functions. Nanomaterials 2019, 9, 1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constantin, C.; Pisani, A.; Bardi, G.; Neagu, M. Nano-carriers of COVID-19 vaccines: The main pillars of efficacy. Nanomedicine 2021, 16, 2377–2387. [Google Scholar] [CrossRef] [PubMed]
- Pisani, A.; Pompa, P.P.; Bardi, G. Potential Applications of Nanomaterials to Quench the Cytokine Storm in Coronavirus Disease 19. Front. Bioeng. Biotechnol 2020, 8, 906. [Google Scholar] [PubMed]
- Stone, C.A.; Liu, Y.; Relling, M.V.; Krantz, M.S.; Pratt, A.L.; Abreo, A.; Hemler, J.A.; Phillips, E.J. Immediate Hypersensitivity to Polyethylene Glycols and Polysorbates: More Common Than We Have Recognized. J. Allergy Clin. Immunol. Pract. 2018, 7, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Gatto, F.; Bardi, G. Metallic nanoparticles: General research approaches to immunological characterization. Nanomaterials 2018, 8, 753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisani, A.; Bardi, G. Immunology of biodegradable nanoparticles: A brief overview on a wide growing field. Explor. Immunol. 2021, 1, 48–60. [Google Scholar] [CrossRef]
- Pisani, A.; Donno, R.; Gennari, A.; Cibecchini, G.; Catalano, F.; Marotta, R.; Pompa, P.P.; Tirelli, N.; Bardi, G. CXCL12-PLGA/Pluronic Nanoparticle Internalization Abrogates CXCR4-Mediated Cell Migration. Nanomater 2020, 10, 2304. [Google Scholar] [CrossRef] [PubMed]
- Vasilichin, V.A.; Tsymbal, S.A.; Fakhardo, A.F.; Anastasova, E.I.; Marchenko, A.S.; Shtil, A.A.; Vinogradov, V.V.; Koshel, E.I. Effects of Metal Oxide Nanoparticles on Toll-Like Receptor mRNAs in Human Monocytes. Nanomaterials 2020, 10, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-halifa, S.; Zottig, X.; Babych, M.; Côté-cyr, M.; Bourgault, S.; Archambault, D. Harnessing the Activation of Toll-Like Receptor 2/6 by Self-Assembled Cross-β Fibrils to Design Adjuvanted Nanovaccines. Nanomater 2020, 10, 1981. [Google Scholar] [CrossRef] [PubMed]
- Ledezma, D.K.; Balakrishnan, P.B.; Cano-Mejia, J.; Sweeney, E.E.; Hadley, M.; Bollard, C.M.; Villagra, A.; Fernandes, R. Indocyanine green-nexturastat A-PLGA nanoparticles combine photothermal and epigenetic therapy for melanoma. Nanomaterials 2020, 10, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremi, I.; Havaki, S.; Georgitsopoulou, S.; Lagopati, N.; Georgakilas, V.; Gorgoulis, V.G.; Georgakilas, A.G. A guide for using transmission electron microscopy for studying the radiosensitizing effects of gold nanoparticles in vitro. Nanomaterials 2021, 11, 859. [Google Scholar] [CrossRef] [PubMed]
- Lohcharoenkal, W.; Abbas, Z.; Rojanasakul, Y. Advances in Nanotechnology-Based Biosensing of Immunoregulatory Cytokines. Biosensors 2021, 11, 364. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardi, G.; Neagu, M. Moving Forward in Nano-Immune Interactions. Nanomaterials 2022, 12, 2033. https://doi.org/10.3390/nano12122033
Bardi G, Neagu M. Moving Forward in Nano-Immune Interactions. Nanomaterials. 2022; 12(12):2033. https://doi.org/10.3390/nano12122033
Chicago/Turabian StyleBardi, Giuseppe, and Monica Neagu. 2022. "Moving Forward in Nano-Immune Interactions" Nanomaterials 12, no. 12: 2033. https://doi.org/10.3390/nano12122033