Tuning Multicolor Emission of Manganese-Activated Gallogermanate Nanophosphors by Regulating Mn Ions Occupying Sites for Multiple Anti-Counterfeiting Application
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Sample Preparation
2.3. Inks Preparation and Anti-Counterfeiting Patterns
2.4. Characterization
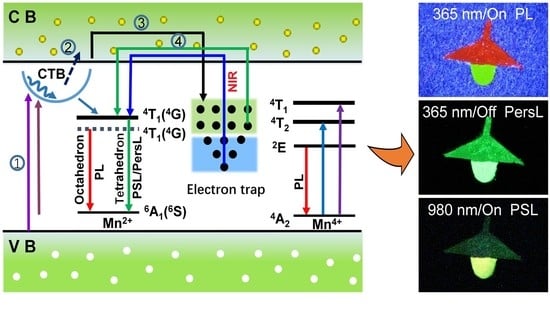
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thejas, K.K.; Abraham, M.; Kunti, A.K.; Tchernycheva, M.; Ahmad, S.; Das, S. Review on deep red-emitting rare-earth free germanates and their efficiency as well as adaptability for various applications. Appl. Mater. Today 2021, 24, 101094. [Google Scholar] [CrossRef]
- Wang, T.; Wang, S.F.; Liu, Z.Y.; He, Z.Y.; Yu, P.; Zhao, M.Y.; Zhang, H.X.; Lu, L.F.; Wang, Z.; Wang, Z.; et al. A hybrid erbium(III)–bacteriochlorin near-infrared probe for multiplexed biomedical imaging. Nat. Mater. 2021, 20, 1571–1578. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, X.H.; Zhang, Y. Exploring heterostructured upconversion nanoparticles: From rational engineering to diverse applications. ACS Nano 2021, 15, 3709–3735. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.Y.; Shi, W.Y.; Lu, C. Control of multicolor and white emission by triplet energy transfer. J. Phys. Chem. A 2021, 125, 4209–4215. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.C.; Bai, W.H.; Xuan, T.T.; Zhou, T.L.; Dong, G.Y.; Xie, R.J. In situ inkjet printing patterned lead halide perovskite quantum dot color conversion films by using cheap and eco-friendly aqueous inks. Small Methods 2021, 5, 2000889. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.W.; Lu, Y.Y.; Liu, F. Sunlight-activated long-persistent luminescence in the near-infrared from Cr3+-doped zinc gallogermanates. Nat. Mater. 2012, 11, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.J.; Zhen, Z.P.; Zhang, F.; Liu, F.; Mishra, J.P.; Tang, W.; Chen, H.M.; Huang, X.L.; Wang, L.C.; Chen, X.Y.; et al. Photostimulable near-infrared persistent luminescent nanoprobes for ultrasensitive and longitudinal deep-tissue bio-imaging. Theranostics 2014, 4, 1112–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, D.L.; Gao, J.; Gao, F.; Kuang, Q.Q.; Pan, Y.; Chen, Y.F.; Pan, Z.W. Quintuple-mode dynamic anti-counterfeiting using multi-mode persistent phosphors. J. Mater. Chem. C 2021, 9, 16634–16644. [Google Scholar] [CrossRef]
- Zhou, Q.; Dolgov, L.; Srivastava, A.M.; Zhou, L.; Wang, Z.L.; Shi, J.X.; Dramićanin, M.D.; Brik, M.G.; Wu, M.M. Mn2+ and Mn4+ red phosphors: Synthesis, luminescence and applications in WLEDs. A review. J. Mater. Chem. C 2018, 6, 2652–2671. [Google Scholar] [CrossRef]
- Kanzaki, M. Crystal structures of Zn2GeO4 cubic/tetragonal spinel and Zn2SiO4 modified spinel phases. J. Miner. Petrol. Sci. 2018, 113, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Gao, D.L.; Ma, K.W.; Wang, P.; Zhang, X.Y.; Pang, Q.; Xin, H.; Zhang, Z.H.; Jiao, H. Tuning multicolour emission of Zn2GeO4: Mn phosphors by Li+ doping for information encryption and anti-counterfeiting applications. Dalton Trans. 2022, 51, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.P.; Zhang, L.; Jia, Y.C.; Xu, Y.H.; Yin, S.W.; You, H.P. ZnGa2−yAlyO4: Mn2+, Mn4+ thermochromic phosphors: Valence state control and optical temperature sensing. Inorg. Chem. 2020, 59, 15969–15976. [Google Scholar] [CrossRef]
- Chen, X.; Yao, W.; Wang, Q.; Wu, W. Designing multicolor dual-mode lanthanide-doped NaLuF4/Y2O3 composites for advanced anticounterfeiting. Adv. Opt. Mater. 2020, 8, 1901209. [Google Scholar] [CrossRef]
- Wang, Y.J.; Włodarczyk, D.; Brik, M.G.; Barzowska, J.; Shekhovtsov, A.N.; Belikov, K.N.; Paszkowicz, W.; Li, L.; Zhou, X.J.; Suchocki, A. Effect of temperature and high pressure on luminescence properties of Mn3+ ions in Ca3Ga2Ge3O12 single crystals. J. Phys. Chem. C 2021, 125, 5146–5157. [Google Scholar] [CrossRef]
- López, S.; Romero, A.H.; Rodríguez-Hernández, P.; Munoz, A. First-principles study of the high-pressure phase transition in ZnAl2O4 and ZnGa2O4: From cubic spinel to orthorhombic post-spinel structures. Phys. Rev. B 2009, 79, 214103. [Google Scholar] [CrossRef]
- Liu, F.; Liang, Y.; Pan, Z. Detection of up-converted persistent luminescence in the near infrared emitted by the Zn3Ga2GeO8: Cr3+, Yb3+, Er3+ phosphor. Phys. Rev. Lett. 2014, 113, 177401. [Google Scholar] [CrossRef]
- Allix, M.; Chenu, S.; Véron, E.; Poumeyrol, T.; Kouadri-Boudjelthia, E.A.; Alahrache, S.; Porcher, F.; Massiot, D.; Fayon, F. Considerable improvement of long-persistent luminescence in germanium and tin substituted ZnGa2O4. Chem. Mater. 2013, 25, 1600–1606. [Google Scholar] [CrossRef]
- Huang, K.; Dou, X.; Zhang, Y.F.; Gou, X.P.; Liu, J.; Qu, J.L.; Li, Y.; Huang, P.; Han, G. Enhancing light and X-ray charging in persistent luminescence nanocrystals for orthogonal afterglow anti-counterfeiting. Adv. Funct. Mater. 2021, 31, 2009920. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, J.; Zheng, W.; Wang, Q.; Li, Z.H.; Cong, H.J.; Liu, H.J.; Chen, X.Y.; Yuan, Q. Controlling disorder in host lattice by hetero-valence ion doping to manipulate luminescence in spinel solid solution phosphors. Sci. China Chem. 2018, 61, 1624–1629. [Google Scholar] [CrossRef]
- Srivastava, B.B.; Gupta, S.K.; Li, Y.; Mao, Y. Bright persistent green emitting water-dispersible Zn2GeO4: Mn nanorods. Dalton Trans. 2020, 49, 7328–7340. [Google Scholar] [CrossRef]
- Ma, Z.D.; Zhou, J.Y.; Zhang, J.C.; Zeng, S.H.; Zhou, H.; Smith, A.T.; Wang, W.X.; Sun, L.Y.; Wang, Z.F. Mechanics-induced triple-mode anticounterfeiting and moving tactile sensing by simultaneously utilizing instantaneous and persistent mechanoluminescence. Mater. Horizons 2019, 6, 2003–2008. [Google Scholar] [CrossRef]
- Lin, S.S.; Lin, H.; Ma, C.G.; Cheng, Y.; Ye, S.Z.; Lin, F.L.; Li, R.F.; Xu, J.; Wang, Y.S. High-security-level multi-dimensional optical storage medium: Nanostructured glass embedded with LiGa5O8: Mn2+ with photostimulated luminescence. Light Sci. Appl. 2020, 9, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, L.; Zhang, L.; Jia, Y.; Shao, B.; Lü, W.; Zhao, S.; You, H. Enhancing luminescence and controlling the Mn valence state of Gd3Ga5−x−δAlx−y+δO12:yMn phosphors by the design of the garnet structure. ACS Appl. Mater. Interfaces 2020, 12, 7334–7344. [Google Scholar] [CrossRef] [PubMed]
- Du, J.R.; Li, K.; Deun, R.V.; Poelman, D.; Lin, H.W. Near-infrared persistent luminescence and trap reshuffling in Mn4+ doped alkali-earth metal tungstates. Adv. Opt. Mater. 2022, 10, 2101714. [Google Scholar] [CrossRef]
- Liu, F.; Yan, W.Z.; Chuang, Y.-J.; Zhen, Z.P.; Xie, J.; Pan, Z.W. Photostimulated near-infrared persistent luminescence as a new optical read-out from Cr3+ doped LiGa5O8. Sci. Rep. 2013, 3, 1554. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.H.; Shi, J.P.; Fu, X.Y.; Wang, C.C.; Sun, X.; Chen, C.J.; Zhuang, Y.X.; Zou, X.Y.; Li, Y.C.; Zhang, H.W. X-ray recharged long afterglow luminescent nanoparticles MgGeO3: Mn2+, Yb3+, Li+ in the first and second biological windows for long-term bioimaging. Nanoscale 2020, 12, 14037–14046. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Huang, K.; Huang, L.; Tian, X.; Dong, H.; Kang, R.; Hu, Y.; Nie, J.; Qiu, J.; et al. Trap energy upconversion-like near-infrared to near-infrared light rejuvenateable persistent luminescence. Adv. Mater. 2021, 33, 2008722. [Google Scholar] [CrossRef]
- Adachi, S. Review—Mn4+-activated red and deep red-emitting phosphors. ECS J. Solid State Sci. Technol. 2020, 9, 016001. [Google Scholar] [CrossRef]
- Brik, M.G.; Camardello, S.J.; Srivastava, A.M. Influence of covalency on the Mn4+ 2Eg→4A2g emission energy in crystals. ECS J. Solid State Sci. Technol. 2015, 4, R39. [Google Scholar] [CrossRef]
- Brik, M.G.; Srivastava, A.M. Critical review—A review of the electronic structure and optical properties of ions with d3 electron configuration (V2+, Cr3+, Mn4+, Fe5+) and main related misconceptions. ECS J. Solid State Sci. Technol. 2017, 7, R3079. [Google Scholar] [CrossRef]
- Si, T.; Zhu, Q.; Xiahou, J.Q.; Sun, X.D.; Li, J.-G. Regulating Mn2+/Mn4+ activators in ZnGa2O4 via Mg2+/Ge4+ doping to generate multimode luminescence for advanced anti-counterfeiting. ACS Appl. Electron. Mater. 2021, 3, 2005–2016. [Google Scholar] [CrossRef]
- Sun, X.Y.; Zhang, J.H.; Zhang, X.; Luo, Y.S.; Hao, Z.D.; Wang, X.J. Effect of retrapping on photostimulated luminescence in Sr3SiO5: Eu2+, Dy3+ phosphor. J. Appl. Phys. 2009, 105, 013501. [Google Scholar] [CrossRef] [Green Version]
- Ueda, J.; Maki, R.; Tanabe, S. Vacuum referred binding energy (VRBE)-guided design of orange persistent Ca3Si2O7: Eu2+ phosphors. Inorg. Chem. 2017, 56, 10353–10360. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.L.; Gao, J.; Zhao, D.; Pang, Q.; Xiao, G.Q.; Wang, L.L.; Ma, K.W. Enhancing the red upconversion luminescence of hybrid porous microtubes via an in situ O-substituted reaction through heat treatment. J. Mater. Chem. C 2020, 8, 17318–17324. [Google Scholar] [CrossRef]
- Gao, D.L.; Zhao, D.; Pan, Y.; Chai, R.P.; Pang, Q.; Zhang, X.Y.; Chen, W. Extending the color response range of Yb3+ concentration-dependent multimodal luminescence in Yb/Er doped fluoride microrods by annealing treatment. Ceram. Int. 2021, 47, 32000–32007. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Q.Q.; Zheng, W.; Liu, H.Y.; Yin, C.Q.; Wang, F.B.; Chen, X.Y.; Yuan, Q.; Tan, W.H. One-dimensional luminous nanorods featuring tunable persistent luminescence for autofluorescence-free biosensing. ACS Nano 2017, 11, 8185–8191. [Google Scholar] [CrossRef]
- You, W.W.; Tu, D.T.; Li, R.F.; Zheng, W.; Chen, X.Y. “Chameleon-like” optical behavior of lanthanide-doped fluoride nanoplates for multilevel anti-counterfeiting applications. Nano Res. 2019, 12, 1417–1422. [Google Scholar] [CrossRef]
- Ding, M.Y.; Dong, B.; Lu, Y.; Yang, X.F.; Yuan, Y.J.; Bai, W.F.; Wu, S.T.; Ji, Z.Z.; Lu, C.H.; Zhang, K.; et al. Energy manipulation in lanthanide-doped core-shell nanoparticles for tunable dual-mode luminescence toward advanced anti-counterfeiting. Adv. Mater. 2020, 32, 2002121. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, R.; Li, H.L.; Lin, Z.X.; Hou, D.J.; Guo, Y.Q.; Song, J.; Song, C.; Lin, Z.W.; Zhang, W.X.; et al. Triple-mode emissions with invisible near-infrared after-glow from Cr3+-doped zinc aluminum germanium nanoparticles for advanced anti-counterfeiting applications. Small 2020, 16, 2003121. [Google Scholar] [CrossRef]
- Ma, L.; Zou, X.; Bui, B.; Chen, W.; Song, K.H.; Solberg, T. X-ray excited ZnS:Cu,Co afterglow nanoparticles for photodynamic activation. Appl. Phys. Lett. 2014, 105, 13702. [Google Scholar] [CrossRef]
- Chen, W.; Wang, S.P.; Westcott, S.; Liu, Y. Dose Dependent X-Ray Luminescence in MgF2: Eu2+, Mn2+ Phosphors. J. Appl. Phys. 2008, 103, 113103. [Google Scholar] [CrossRef]
- Wang, S.P.; Westcott, L.S.; Chen, W. Nanoparticle Luminescence Thermometry. J. Phys. Chem. B 2002, 106, 11203–11209. [Google Scholar] [CrossRef]
- Chen, W.; Joly, A.; Malm, J.O.; Bovin, J.O.; Wang, S.P. Full-Color Emission and Temperature Dependence of The Luminescence In Poly-P-Phenylene Ethynylene-ZnS:Mn2+ Composite Particles. J. Phys. Chem. B 2003, 107, 6544–6551. [Google Scholar] [CrossRef]
- Li, Y.; Lu, W.; Huang, Q.; Huang, M.; Li, C.; Chen, W. Copper sulfide nanoparticles for photothermal ablation of tumor cells. Nanomedicine 2010, 5, 1161–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakshmanan, S.B.; Zou, X.; Hossu, M.; Ma, L.; Yang, C.; Chen, W. Local field enhanced Au/CuS nanocomposites as efficient photothermal transducer agents for cancer treatment. J. Biomed. Nanotechnol. 2012, 8, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Li, K.; Guo, H.; Zheng, H.; Shuda, S.; Wang, X.; Zhang, J.; Chen, W.; Zhang, Y. Exploration of Graphitic-C3N4 Quantum Dots for Microwave-Induced Photodynamic Therapy. ACS Biomater. Sci. Eng. 2017, 3, 1836–1844. [Google Scholar] [CrossRef]
- Ma, L.; Chen, W. ZnS:Cu,Co water-soluble afterglow nanoparticles: Synthesis, luminescence and potential applications. Nanotechnology 2010, 21, 385604. [Google Scholar] [CrossRef]
- Tang, X.; Yu, H.; Bui, B.; Wang, L.; Xing, C.; Wang, S.; Chen, M.; Hu, Z.; Chen, W. Nitrogen-doped fluorescence carbon dots as multi-mechanism detection for iodide and curcumin in biological and food samples. Bioact. Mater. 2020, 6, 1541–1554. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, D.; Wang, P.; Gao, F.; Nguyen, W.; Chen, W. Tuning Multicolor Emission of Manganese-Activated Gallogermanate Nanophosphors by Regulating Mn Ions Occupying Sites for Multiple Anti-Counterfeiting Application. Nanomaterials 2022, 12, 2029. https://doi.org/10.3390/nano12122029
Gao D, Wang P, Gao F, Nguyen W, Chen W. Tuning Multicolor Emission of Manganese-Activated Gallogermanate Nanophosphors by Regulating Mn Ions Occupying Sites for Multiple Anti-Counterfeiting Application. Nanomaterials. 2022; 12(12):2029. https://doi.org/10.3390/nano12122029
Chicago/Turabian StyleGao, Dangli, Peng Wang, Feng Gao, William Nguyen, and Wei Chen. 2022. "Tuning Multicolor Emission of Manganese-Activated Gallogermanate Nanophosphors by Regulating Mn Ions Occupying Sites for Multiple Anti-Counterfeiting Application" Nanomaterials 12, no. 12: 2029. https://doi.org/10.3390/nano12122029