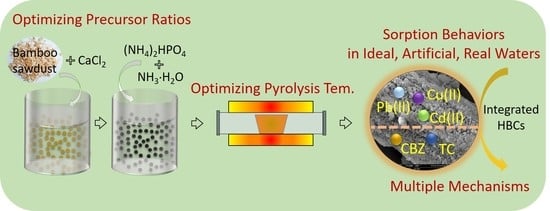
Integration of Micro-Nano-Engineered Hydroxyapatite/Biochars with Optimized Sorption for Heavy Metals and Pharmaceuticals
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials and Reagents
2.2. Preparation of Hydroxyapatite-Modified, Bamboo-Based Biochar
2.3. Characterization of HBCs
2.4. Batch Experiments and Data Analysis
3. Results and Discussion
3.1. Characterization of HBCs
3.2. Sorption of Cu(II), Cd(II), and Pb(II)
3.2.1. Sorption Kinetics
3.2.2. Effects of Solution pH, Ionic Strength, and Coexisting Cation, Anion, and HA
3.2.3. Sorption Isotherms and Thermodynamic Study
3.3. Sorption of CBZ and TC
3.3.1. Sorption Kinetics
3.3.2. Effects of Solution pH and Ionic Strength
3.3.3. Sorption Isotherms and Thermodynamic Investigation
3.4. Reusability and Sorption in Simulated Wastewater
3.5. Potential Sorption Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, B.; Yang, L. A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem. J. 2010, 94, 99–107. [Google Scholar] [CrossRef]
- Cheng, S. Heavy metal pollution in China: Origin, pattern and control. Environ. Sci. Pollut. Res. 2003, 10, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, T.; Hsu, P.C.; Xie, J.; Zhao, J.; Liu, K.; Sun, J.; Xu, J.; Tang, J.; Ye, Z.; et al. Direct/alternating current electrochemical method for removing and recovering heavy metal from water using graphene oxide electrode. ACS Nano 2019, 13, 6431–6437. [Google Scholar] [CrossRef] [PubMed]
- John, R.; Ahmad, P.; Gadgil, K.; Sharma, S. Heavy metal toxicity: Effect on plant growth, biochemical parameters and metal accumulation by Brassica juncea L. Int. J. Plant Prod. 2012, 3, 65–76. [Google Scholar]
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. 2008, 152, 686–692. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; WHO Library Cataloguing-in-Publication Data: Geneva, Switzerland, 2011. [Google Scholar]
- Gadipelly, C.; Pérez-González, A.; Yadav, G.D.; Ortiz, I.; Ibáñez, R.; Rathod, V.K.; Marathe, K.V. Pharmaceutical industry wastewater: Review of the technologies for water treatment and reuse. Ind. Eng. Chem. Res. 2014, 53, 11571–11592. [Google Scholar] [CrossRef]
- Ali, H.A.; Yaniv, K.; Bar-Zeev, E.; Chaudhury, S.; Shagan, M.; Lakkakula, S.; Ronen, Z.; Kushmaro, A.; Nir, O. Tracking SARS-CoV-2 RNA through the wastewater treatment process. ACS ES&T Water 2021, 1, 1161–1167. [Google Scholar]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U., Jr.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Kang, R.; Yin, Y.; Tu, S.; Ye, L. Two-step pyrolysis biochar derived from agro-waste for antibiotics removal: Mechanisms and stability. Chemosphere 2022, 292, 133454. [Google Scholar] [CrossRef]
- Shan, D.; Deng, S.; Zhao, T.; Wang, B.; Wang, Y.; Huang, J.; Yu, G.; Winglee, J.; Wiesner, M.R. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling. J. Hazard. Mater. 2016, 305, 156–163. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.D.A.; Akhtar, A.; Nabi, S.A. Kinetics and thermodynamics of alkaline earth and heavy metal ion exchange under particle diffusion controlled phenomenon using polyaniline-Sn(IV)iodophosphate nanocomposite. J. Chem. Eng. Data 2014, 59, 2677–2685. [Google Scholar] [CrossRef]
- Busca, G. Bases and basic materials in industrial and environmental chemistry: A review of commercial processes. Ind. Eng. Chem. Res. 2009, 48, 6486–6511. [Google Scholar] [CrossRef]
- Lei, Y.; Zhan, Z.; Saakes, M.; van der Weijden, R.D.; Buisman, C.J.N. Electrochemical recovery of phosphorus from acidic cheese wastewater: Feasibility, quality of products, and comparison with chemical precipitation. ACS ES&T Water 2021, 1, 1002–1013. [Google Scholar]
- Su, X.; Kushima, A.; Halliday, C.; Zhou, J.; Li, J.; Hatton, T.A. Electrochemically-mediated selective capture of heavy metal chromium and arsenic oxyanions from water. Nat. Commun. 2018, 9, 4701. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wang, L.; Zhang, Z.; Zhang, Y.; Zhu, J.; Wang, X.; Lv, Y.; Miao, R. Stable forward osmosis nanocomposite membrane doped with sulfonated grapheme oxide@metal-organic frameworks for heavy metal removal. ACS Appl. Mater. Interfaces 2020, 12, 57102–57116. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xiao, J.; Yang, X.; Zhao, Y.; Hu, R.; Ding, J.; Gong, Y.; Tian, X. New insights into nanostructure/functionality-dependent catalysis of pollutants by arc-designing graphite-encapsulated silver nanoparticles. Chem. Eng. J. 2022, 430, 132774. [Google Scholar] [CrossRef]
- Keen, O.S.; Baik, S.; Linden, K.G.; Aga, D.S.; Love, N.G. Enhanced biodegradation of carbamazepine after UV/H2O2 advanced oxidation. Environ. Sci. Technol. 2012, 46, 6222–6227. [Google Scholar] [CrossRef]
- Ali, I. New generation adsorbents for water treatment. Chem. Rev. 2012, 112, 5073–5091. [Google Scholar] [CrossRef]
- Ramlogan, M.V.; Rabinovich, A.; Rouff, A.A. Thermochemical analysis of ammonia gas sorption by struvite from livestock wastes and comparison with biochar and metal-organic framework sorbents. Environ. Sci. Technol. 2020, 54, 13264–13273. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, C.; Huang, A. EDTA-functionalized covalent organic framework for the removal of heavy-metal ions. ACS Appl. Mater. Interfaces 2019, 11, 32186–32191. [Google Scholar] [CrossRef]
- Li, S.; Wang, W.; Liang, F.; Zhang, W. Heavy metal removal using nanoscale zero-valent iron (nZVI): Theory and application. J. Hazard. Mater. 2017, 322, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Xiao, J.; Wang, T.; Chen, G.; Chen, L.; Tian, X. Engineering of phosphate-functionalized biochars with highly developed surface area and porosity for efficient and selective extraction of uranium. Chem. Eng. J. 2020, 379, 122388. [Google Scholar] [CrossRef]
- Li, K.; Wang, X. Adsorptive removal of Pb(II) by activated carbon prepared from Spartina alterniflora: Equilibrium, kinetics and thermodynamics. Bioresour. Technol. 2009, 100, 2810–2815. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Deva, D.; Sharma, A.; Verma, N. Fe-grown carbon nanofibers for removal of arsenic(V) in wastewater. Ind. Eng. Chem. Res. 2010, 49, 7074–7084. [Google Scholar] [CrossRef]
- Wang, F.; Yao, J.; Liu, H.; Liu, R.; Chen, H.; Yi, Z.; Yu, Q.; Ma, L.; Xing, B. Cu and Cr enhanced the effect of various carbon nanotubes on microbial communities in an aquatic environment. J. Hazard. Mater. 2015, 292, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liang, F.; Hu, W.; Yang, X.; Xiang, H.; Wang, G.; Fei, B.; Liu, Z. Economic analysis of a hypothetical bamboo-biochar plant in Zhejiang Province, China. Waste Manag. Res. 2017, 35, 1220–1225. [Google Scholar] [CrossRef]
- Meyer, S.; Glaser, B.; Quicker, P. Technical, economical, and climate-related aspects of biochar production technologies: A literature review. Environ. Sci. Technol. 2011, 45, 9473–9483. [Google Scholar] [CrossRef]
- Sizmur, T.; Fresno, T.; Akgül, G.; Frost, H.; Moreno-Jiménez, E. Biochar modification to enhance sorption of inorganics from water. Bioresour. Technol. 2017, 246, 34–47. [Google Scholar] [CrossRef]
- Godwin, P.M.; Pan, Y.; Xiao, H.; Afzal, M.T. Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. J. Bioresour. Bioprod. 2019, 4, 31–42. [Google Scholar] [CrossRef]
- Huang, H.L.; Huang, Z.H.; Chu, Y.C.; Lin, H.P.; Chang, Y.J. Application of metallic nanoparticle-biochars with ionic liquids for thermal transfer fluids. Chemosphere 2020, 250, 126219. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.C.; Jiang, J.; Hu, J.; Hu, X.J.; Yang, Q.; Zhou, S.Q. Removal of Pb(II) from aqueous solution by hydroxyapatite/carbon composite: Preparation and adsorption behavior. Colloid. Surf. A 2019, 577, 471–479. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, X.; Wang, T.; Hu, R.; Wu, J. Oxygen-functionalized Typha angustifolia biochars derived from various pyrolysis temperatures: Physicochemical properties, heavy metal capture behaviors and mechanism. Colloid. Surf. A 2021, 628, 127259. [Google Scholar] [CrossRef]
- Son, E.B.; Poo, K.M.; Chang, J.S.; Chae, K.J. Heavy metal removal from aqueous solutions using engineered magnetic biochars derived from waste marine macro-algal biomass. Sci. Total Environ. 2018, 615, 161–168. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Y.; Wang, H.; Lu, W.; Zhou, Z.; Zhang, Y.; Ren, L. Influence of pyrolysis temperature on characteristics and heavy metal adsorptive performance of biochar derived from municipal sewage sludge. Bioresour. Technol. 2014, 164, 47–54. [Google Scholar] [CrossRef]
- Jung, K.W.; Lee, S.Y.; Choi, J.W.; Lee, Y.J. A facile one-pot hydrothermal synthesis of hydroxyapatite/biochar nanocomposites: Adsorption behavior and mechanisms for the removal of copper(II) from aqueous media. Chem. Eng. J. 2019, 369, 529–541. [Google Scholar] [CrossRef]
- Uchimiya, M.; Bannon, D.I.; Wartelle, L.H.; Lima, I.M.; Klasson, K.T. Lead retention by broiler litter biochars in small arms range soil: Impact of pyrolysis temperature. J. Agric. Food Chem. 2012, 60, 5035–5044. [Google Scholar] [CrossRef]
- Hu, R.; Xiao, J.; Wang, T.; Gong, Y.; Chen, G.; Chen, L.; Tian, X. Highly concentrated amino-modified biochars using a plasma: Evolution of surface composition and porosity for heavy metal capture. Carbon 2020, 168, 515–527. [Google Scholar] [CrossRef]
- Bekiaris, G.; Peltre, C.; Jensen, L.S.; Bruun, S. Using FTIR-photoacoustic spectroscopy for phosphorus speciation analysis of biochars. Spectrochim. Acta A 2016, 168, 29–36. [Google Scholar] [CrossRef]
- Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical models of sorption kinetics including a surface reaction mechanism: A review. Adv. Colloid Interface Sci. 2009, 152, 2–13. [Google Scholar] [CrossRef]
- Mercer, K.L.; Tobiason, J.E. Removal of arsenic from high ionic strength solutions: Effects of ionic strength, pH, and preformed versus in situ formed HFO. Environ. Sci. Technol. 2008, 42, 3797–3802. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, L.; Chen, Y.; Fang, M.; Zhang, J.; Wang, H. Highly efficient, irreversible and selective ion exchange property of layered titanate nanostructures. Adv. Funct. Mater. 2012, 22, 835–841. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Khan, M.A.; Otero, M.; Siddiqui, M.R.; Jeon, B.H.; Batoo, K.M. A magnetic nanocomposite produced from camel bones for an efficient adsorption of toxic metals from water. J. Clean. Prod. 2018, 178, 293–304. [Google Scholar] [CrossRef]
- Cheng, T.W.; Lee, M.L.; Ko, M.S.; Ueng, T.H.; Yang, S.F. The heavy metal adsorption characteristics on metakaolin-based geopolymer. Appl. Clay Sci. 2012, 5, 90–96. [Google Scholar] [CrossRef]
- Nielsen, L.; Biggs, M.J.; Skinner, W.; Bandosz, T.J. The effects of activated carbon surface features on the reactive adsorption of carbamazepine and sulfamethoxazole. Carbon 2014, 80, 419–432. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Deng, S.; Huang, Q.; Nie, Y.; Wang, B.; Huang, J.; Yu, G. Regenerable granular carbon nanotubes/alumina hybrid adsorbents for diclofenac sodium and carbamazepine removal from aqueous solution. Water Res. 2013, 47, 4139–4147. [Google Scholar] [CrossRef]
- Jiao, S.; Zheng, S.; Yin, D.; Wang, L.; Chen, L. Aqueous photolysis of tetracycline and toxicity of photolytic products to luminescent bacteria. Chemosphere 2008, 73, 377–382. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, B.; Zhu, Z.; Liu, H.; Huang, Y.; Zhao, X.; Liang, M. Characterization, dissolution and solubility of the hydroxypyromorphite-hydroxyapatite solid solution [(PbxCa1-x)5(PO4)3OH] at 25 °C and pH 2-9. Geochem. T 2016, 17, 2. [Google Scholar] [CrossRef] [Green Version]
- Mu, Y.; Saffarzadeh, A.; Shimaoka, T. Feasibility of using natural fishbone apatite on removal of Pb from municipal solid waste incineration (MSWI) fly ash. Procedia Environ. Sci. 2016, 31, 345–350. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Lai, C.; Zeng, G.; Huang, D.; Yang, C.; Wang, Y.; Zhou, Y.; Cheng, M. Efficacy of carbonaceous nanocomposites for sorbing ionizable antibiotic sulfamethazine from aqueous solution. Water Res. 2016, 95, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, D.; Xie, S.; Quan, H.; Luo, X.; Guo, L. Adsorption behaviors of organic micropollutants on zirconium metal–organic framework UiO-66: Analysis of surface interactions. ACS Appl. Mater. Interfaces 2017, 9, 41043–41054. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Yuan, P.; Yang, Z.; Peng, W.; Meng, X.; Cheng, J. Integration of Micro-Nano-Engineered Hydroxyapatite/Biochars with Optimized Sorption for Heavy Metals and Pharmaceuticals. Nanomaterials 2022, 12, 1988. https://doi.org/10.3390/nano12121988
Zhao X, Yuan P, Yang Z, Peng W, Meng X, Cheng J. Integration of Micro-Nano-Engineered Hydroxyapatite/Biochars with Optimized Sorption for Heavy Metals and Pharmaceuticals. Nanomaterials. 2022; 12(12):1988. https://doi.org/10.3390/nano12121988
Chicago/Turabian StyleZhao, Xin, Peiling Yuan, Ziyan Yang, Wei Peng, Xiang Meng, and Jiang Cheng. 2022. "Integration of Micro-Nano-Engineered Hydroxyapatite/Biochars with Optimized Sorption for Heavy Metals and Pharmaceuticals" Nanomaterials 12, no. 12: 1988. https://doi.org/10.3390/nano12121988