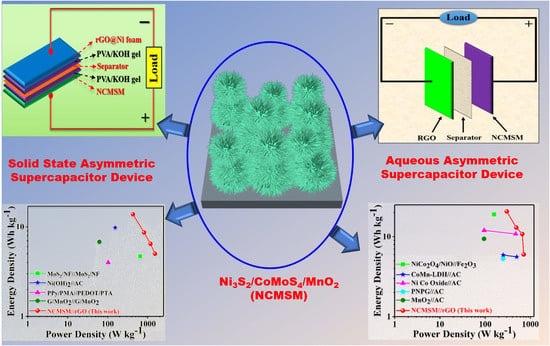
Ternary Nanohybrid of Ni3S2/CoMoS4/MnO2 on Nickel Foam for Aqueous and Solid-State High-Performance Supercapacitors
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Materials
2.1.1. Materials
2.1.2. Preparation of Ni3S2/CoMoS4@Ni Foam (NCMS)
2.1.3. Preparation of Ni3S2/CoMoS4/MnO2@Ni Foam (NCMSM)
2.1.4. Preparation of Ni3S2@Ni Foam (NS)
2.1.5. Asymmetric SC Devices Fabrication
2.1.6. Characterization
3. Results and Discussion
3.1. Synthesis and Structural Analysis
3.2. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, J.; Wan, L.; Yang, S.; Xiao, F.; Wang, S. Design hierarchical electrodes with highly conductive NiCo2S4 nanotube arrays grown on carbon fiber paper for high-performance pseudocapacitors. Nano Lett. 2014, 14, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xia, C.; Alshareef, H.N. One-step electrodeposited nickel-cobalt sulfide nanosheet arrays for high-performance asymmetric supercapacitors. ACS Nano 2014, 8, 9531–9541. [Google Scholar] [CrossRef]
- Hussain, I.; Lamiel, C.; Mohamed, S.G.; Vijayakumar, S.; Ali, A.; Shim, J.-J. Controlled synthesis and growth mechanism of zinc cobalt sulfide rods on Ni-foam for high-performance supercapacitors. J. Ind. Eng. Chem. 2019, 71, 250–259. [Google Scholar] [CrossRef]
- Chen, X.; Ding, Z.; Yu, H.; Ge, H.; Liu, W.; Sun, S. Facile fabrication of CuCo2S4 nanoparticles/MXene composite as anode for high-performance asymmetric supercapacitor. Mater. Chem. Front. 2021, 5, 7606–7616. [Google Scholar] [CrossRef]
- Suo, G.; Ahmed, S.M.; Cheng, Y.; Zhang, J.; Li, Z.; Hou, X.; Yang, Y.; Ye, X.; Feng, L.; Zhang, L. Heterostructured CoS2/CuCo2S4@ N-doped carbon hollow sphere for potassium-ion batteries. J. Colloid Interface Sci. 2022, 608, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Liu, H.; Bi, S.; Gao, C.; Meng, X.; Wang, Y. Insight on the conversion reaction mechanism of NiCo2S4@CNTs as anode materials for lithium-ion batteries and sodium ion batteries. Electrochim. Acta 2021, 388, 138618. [Google Scholar] [CrossRef]
- Jin, D.; Li, Z.; Wang, Z. Hierarchical NiCo2O4 and NiCo2S4 nanomaterials as electrocatalysts for methanol oxidation reaction. Int. J. Hydrog. Energy 2021, 46, 32069–32080. [Google Scholar] [CrossRef]
- Yu, X.Y.; Lou, X.W. Mixed metal sulfides for electrochemical energy storage and conversion. Adv. Energy Mater. 2018, 8, 1701592. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, G.; Zhao, W.; Chen, Y.; Yang, Y.; Huang, L.; Pan, D. Versatile and low-toxic solution approach to binary, ternary, and quaternary metal sulfide thin films and its application in Cu2ZnSn (S, Se) 4 solar cells. Chem. Mater. 2014, 26, 3098–3103. [Google Scholar] [CrossRef]
- Sahoo, S.; Shim, J.-J. Facile synthesis of three-dimensional ternary ZnCo2O4/reduced graphene oxide/NiO composite film on nickel foam for next generation supercapacitor electrodes. ACS Sustain. Chem. Eng. 2017, 5, 241–251. [Google Scholar] [CrossRef]
- Dhakal, G.; Mohapatra, D.; Tamang, T.L.; Lee, M.; Lee, Y.R.; Shim, J.-J. Redox-additive electrolyte–driven enhancement of the electrochemical energy storage performance of asymmetric Co3O4//carbon nano-onions supercapacitors. Energy 2021, 218, 119436. [Google Scholar] [CrossRef]
- Hussain, I.; Mohapatra, D.; Dhakal, G.; Lamiel, C.; Sayed, M.S.; Sahoo, S.; Mohamed, S.G.; Kim, J.S.; Lee, Y.R.; Shim, J.-J. Uniform growth of ZnS nanoflakes for high-performance supercapacitor applications. J. Energy Storage 2021, 36, 102408. [Google Scholar] [CrossRef]
- Mohamed, S.G.; Hussain, I.; Sayed, M.S.; Shim, J.-J. One-step development of octahedron-like CuCo2O4@Carbon fibers for high-performance supercapacitors electrodes. J. Alloys Compd. 2020, 842, 155639. [Google Scholar] [CrossRef]
- Kumar, D.R.; Prakasha, K.; Prakash, A.; Shim, J.-J. Direct growth of honeycomb-like NiCo2O4@ Ni foam electrode for pouch-type high-performance asymmetric supercapacitor. J. Alloys Compd. 2020, 836, 155370. [Google Scholar] [CrossRef]
- Hussain, I.; Ali, A.; Lamiel, C.; Mohamed, S.G.; Sahoo, S.; Shim, J.-J. A 3D walking palm-like core–shell CoMoO4@ NiCo2S4@nickel foam composite for high-performance supercapacitors. Dalton Trans. 2019, 48, 3853–3861. [Google Scholar] [CrossRef]
- Sahoo, S.; Shim, J.-J. Nanostructured 3D zinc cobaltite/nitrogen-doped reduced graphene oxide composite electrode for supercapacitor applications. J. Ind. Eng. Chem. 2017, 54, 205–217. [Google Scholar] [CrossRef]
- Mohamed, S.G.; Hussain, I.; Shim, J.-J. One-step synthesis of hollow C-NiCo2S4 nanostructures for high-performance supercapacitor electrodes. Nanoscale 2018, 10, 6620–6628. [Google Scholar] [CrossRef]
- Phonsuksawang, P.; Khajondetchairit, P.; Ngamchuea, K.; Butburee, T.; Sattayaporn, S.; Chanlek, N.; Suthirakun, S.; Siritanon, T. Enhancing performance of NiCo2S4/Ni3S2 supercapacitor electrode by Mn doping. Electrochim. Acta 2021, 368, 137634. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zhang, B.; Wang, C.; Xia, X.; Lei, W.; Hao, Q. Bimetallic metal-organic framework derived porous NiCo2S4 nanosheets arrays as binder-free electrode for hybrid supercapacitor. Appl. Surf. Sci. 2021, 542, 148621. [Google Scholar] [CrossRef]
- Yang, S.; Xiong, W.; Wu, Z.; Zou, Y.; Huang, H.; Zhou, W.; Cheng, Z.; Liu, D.; Wang, J.; Luo, G. Rational design and synthesis of multi-shelled NiCo2S4 hollow microspheres for high performance supercapacitors. J. Energy Storage 2021, 44, 103407. [Google Scholar] [CrossRef]
- Tang, X.-F.; Yang, Z.-G.; Liang, J.-H. Synthesis of a ternary FeNi2S4/CNT/graphene nanocomposite with improved electrochemical properties. RSC Adv. 2016, 6, 88168–88173. [Google Scholar] [CrossRef]
- Kumar, R.; Rai, P.; Sharma, A. Free-standing NiV2S4 nanosheet arrays on a 3D Ni framework via an anion exchange reaction as a novel electrode for asymmetric supercapacitor applications. J. Mater. Chem. A 2016, 4, 17512–17520. [Google Scholar] [CrossRef]
- Xiao, T.; Che, P.; Xiao, R.; Xiang, P.; Jiang, L.; Tao, F.; Tan, X.; Chen, X. 3D interconnected Fe-Co-S nanosheets network directly grown on graphene coated nickel foam with enhanced electrochemical performance for asymmetric supercapacitors. Appl. Surf. Sci. 2021, 543, 148747. [Google Scholar] [CrossRef]
- Xin, C.; Ang, L.; Musharavati, F.; Jaber, F.; Hui, L.; Zalnezhad, E.; Bae, S.; Hui, K.S.; Hui, K.N. Supercapacitor performance of nickel-cobalt sulfide nanotubes decorated using Ni Co-layered double hydroxide nanosheets grown in situ on Ni foam. Nanomaterials 2020, 10, 584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tung, D.T.; Dung, H.T.; Dung, N.T.; Hong, P.N.; Nguyet, H.M.; Van-Quynh, N.; Van Chuc, N.; Trung, V.Q.; Minh, P.N. Freeze gelation 3D printing of rGO-CuCo2S4 nanocomposite for high-performance supercapacitor electrodes. Electrochim. Acta 2021, 392, 138992. [Google Scholar] [CrossRef]
- Gajraj, V.; Azmi, R.; Indris, S.; Mariappan, C. Boosting the Multifunctional Properties of MnCo2O4-MnCo2S4 Heterostructure for Portable All-Solid-State Symmetric Supercapacitor, Methanol Oxidation and Hydrogen Evolution Reaction. ChemistrySelect 2021, 6, 11466–11481. [Google Scholar] [CrossRef]
- Ramos, M.; Berhault, G.; Ferrer, D.A.; Torres, B.; Chianelli, R.R. HRTEM and molecular modeling of the MoS2–Co9S8 interface: Understanding the promotion effect in bulk HDS catalysts. Catal. Sci. Technol. 2012, 2, 164–178. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.-H.; Kong, L.-B.; Yan, K.; Shi, M.; Zhang, T.; Luo, Y.-C.; Kang, L. Simple synthesis of a CoMoS4 based nanostructure and its application for high-performance supercapacitors. RSC Adv. 2016, 6, 7633–7642. [Google Scholar] [CrossRef]
- Xu, X.; Song, Y.; Xue, R.; Zhou, J.; Gao, J.; Xing, F. Amorphous CoMoS4 for a valuable energy storage material candidate. Chem. Eng. J. 2016, 301, 266–275. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Sun, H.; Zan, P.; Zhao, L.; Lian, J. Growth of vertically aligned Co3S4/CoMo2S4 ultrathin nanosheets on reduced graphene oxide as a high-performance supercapacitor electrode. J. Mater. Chem. A 2016, 4, 18857–18867. [Google Scholar] [CrossRef]
- Van Hoa, N.; Dat, P.A.; Nghia, N.H. One-step preparation of 3D binder-free electrode of porous Co-Mo-S nanostructures grown on Ni foam for supercapacitors. J. Mater. Sci. 2021, 56, 5132–5142. [Google Scholar] [CrossRef]
- Ma, F.; Dai, X.; Jin, J.; Tie, N.; Dai, Y. Hierarchical core-shell hollow CoMoS4@ Ni–Co–S nanotubes hybrid arrays as advanced electrode material for supercapacitors. Electrochim. Acta 2020, 331, 135459. [Google Scholar] [CrossRef]
- Guo, G.; Song, Z.; Cong, C.; Zhang, K. CoMoS4 Nanoflowers as Anode for Secondary Lithium Batteries. J. Nanopart. Res. 2007, 9, 653–656. [Google Scholar] [CrossRef]
- Wang, J.; Chao, D.; Liu, J.; Li, L.; Lai, L.; Lin, J.; Shen, Z. Ni3S2@MoS2 core/shell nanorod arrays on Ni foam for high-performance electrochemical energy storage. Nano Energy 2014, 7, 151–160. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, W.; Nai, J.; Yin, P.; Chen, C.; Guo, L. Selective synthesis of peapodlike Ni/Ni3S2 nanochains and nickel sulfide hollow chains and their magnetic properties. Adv. Funct. Mater. 2010, 20, 3678–3683. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Shim, J.-J. In situ growth of hierarchical mesoporous NiCo2S4@MnO2 arrays on nickel foam for high-performance supercapacitors. Electrochim. Acta 2015, 166, 302–309. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Shim, J.-J. Rapid one-step synthesis and electrochemical properties of graphene/carbon nanotubes/MnO2 composites. Synth. Met. 2015, 199, 276–279. [Google Scholar] [CrossRef]
- Li, F.; Li, J.; Lin, X.; Li, X.; Fang, Y.; Jiao, L.; An, X.; Fu, Y.; Jin, J.; Li, R. Designed synthesis of multi-walled carbon nanotubes@ Cu@MoS2 hybrid as advanced electrocatalyst for highly efficient hydrogen evolution reaction. J. Power Sources 2015, 300, 301–308. [Google Scholar] [CrossRef]
- Polychronopoulou, K.; Malliakas, C.D.; He, J.; Kanatzidis, M.G. Selective surfaces: Quaternary Co (Ni) MoS-based chalcogels with divalent (Pb2+, Cd2+, Pd2+) and trivalent (Cr3+, Bi3+) metals for gas separation. Chem. Mater. 2012, 24, 3380–3392. [Google Scholar] [CrossRef]
- Shen, L.; Wang, J.; Xu, G.; Li, H.; Dou, H.; Zhang, X. NiCo2S4 nanosheets grown on nitrogen-doped carbon foams as an advanced electrode for supercapacitors. Adv. Energy Mater. 2015, 5, 1400977. [Google Scholar] [CrossRef]
- Miao, J.; Xiao, F.-X.; Yang, H.B.; Khoo, S.Y.; Chen, J.; Fan, Z.; Hsu, Y.-Y.; Chen, H.M.; Zhang, H.; Liu, B. Hierarchical Ni-Mo-S nanosheets on carbon fiber cloth: A flexible electrode for efficient hydrogen generation in neutral electrolyte. Sci. Adv. 2015, 1, e1500259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, G.; Liu, Y.; Zhang, L.; Kan, E.; Zhang, S.; Tang, J.; Tang, W. MnO2 nanorods intercalating graphene oxide/polyaniline ternary composites for robust high-performance supercapacitors. Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Guo, S.; Fang, Y.; Han, L.; Wang, E.; Dong, S. One-step electrochemical approach to the synthesis of graphene/MnO2 nanowall hybrids. Nano Res. 2011, 4, 648–657. [Google Scholar] [CrossRef]
- Zhan, S.; Zhu, D.; Qiu, M.; Yu, H.; Li, Y. Highly efficient removal of NO with ordered mesoporous manganese oxide at low temperature. RSC Adv. 2015, 5, 29353–29361. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Fang, C.; Gao, R.; Qian, Y.; Shi, L.; Zhang, J. MnOx–CeOx/CNTs pyridine-thermally prepared via a novel in situ deposition strategy for selective catalytic reduction of NO with NH3. RSC Adv. 2013, 3, 8811–8819. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Shao, H.; Xu, J.; Chen, F.; Feng, Y. Preparation of MoS2 nanofibers by electrospinning. Mater. Lett. 2012, 73, 223–225. [Google Scholar] [CrossRef]
- Han, J.; Li, L.; Fang, P.; Guo, R. Ultrathin MnO2 nanorods on conducting polymer nanofibers as a new class of hierarchical nanostructures for high-performance supercapacitors. J. Phys. Chem. C 2012, 116, 15900–15907. [Google Scholar] [CrossRef]
- Pan, S.; Zhu, J.; Liu, X. Preparation, electrochemical properties, and adsorption kinetics of Ni3S2/graphene nanocomposites using alkyldithiocarbonatio complexes of nickel (ii) as single-source precursors. New J. Chem. 2013, 37, 654–662. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, T.; Li, C.; Ma, J. Hierarchical porous nanostructures assembled from ultrathin MnO2 nanoflakes with enhanced supercapacitive performances. J. Mater. Chem. 2012, 22, 2751–2756. [Google Scholar] [CrossRef]
- Guan, B.; Guo, D.; Hu, L.; Zhang, G.; Fu, T.; Ren, W.; Li, J.; Li, Q. Facile synthesis of ZnCo2O4 nanowire cluster arrays on Ni foam for high-performance asymmetric supercapacitors. J. Mater. Chem. A 2014, 2, 16116–16123. [Google Scholar] [CrossRef]
- Zou, R.; Yuen, M.F.; Zhang, Z.; Hu, J.; Zhang, W. Three-dimensional networked NiCo2O4/MnO2 branched nanowire heterostructure arrays on nickel foam with enhanced supercapacitor performance. J. Mater. Chem. A 2015, 3, 1717–1723. [Google Scholar] [CrossRef]
- Xing, Z.; Chu, Q.; Ren, X.; Ge, C.; Qusti, A.H.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Ni3S2 coated ZnO array for high-performance supercapacitors. J. Power Sources 2014, 245, 463–467. [Google Scholar] [CrossRef]
- Zhang, W.-B.; Kong, L.-B.; Ma, X.-J.; Luo, Y.-C.; Kang, L. Nickel vanadate and nickel oxide nanohybrid on nickel foam as pseudocapacitive electrodes for electrochemical capacitors. RSC Adv. 2014, 4, 41772–41777. [Google Scholar] [CrossRef]
- Min, S.; Zhao, C.; Chen, G.; Qian, X. One-pot hydrothermal synthesis of reduced graphene oxide/Ni(OH)2 films on nickel foam for high performance supercapacitors. Electrochim. Acta 2014, 115, 155–164. [Google Scholar] [CrossRef]
- Fan, H.; Niu, R.; Duan, J.; Liu, W.; Shen, W. Fe3O4@ carbon nanosheets for all-solid-state supercapacitor electrodes. ACS Appl. Mater. Interfaces 2016, 8, 19475–19483. [Google Scholar] [CrossRef]
- Mishra, R.K.; Kushwaha, A.K.; Kim, S.; Seo, S.G.; Jin, S.H. Vertical-slate-like MoS2 nanostructures on 3D-Ni-foam for binder-free, low-cost, and scalable solid-state symmetric supercapacitors. Curr. Appl. Phys. 2019, 19, 1–7. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Kim, J.; Wang, Y.; Huang, H.; Kim, Y. Flexible and wearable fiber shaped high voltage supercapacitors based on copper hexacyanoferrate and porous carbon coated carbon fiber electrodes. J. Mater. Chem. A 2016, 4, 4934–4940. [Google Scholar] [CrossRef]
- Suppes, G.M.; Cameron, C.G.; Freund, M.S. A polypyrrole/phosphomolybdic acid|poly (3,4-ethylenedioxythiophene)/phosphotungstic acid asymmetric supercapacitor. J. Electrochem. Soc. 2010, 157, A1030. [Google Scholar] [CrossRef]
- He, Y.; Chen, W.; Li, X.; Zhang, Z.; Fu, J.; Zhao, C.; Xie, E. Freestanding three-dimensional graphene/MnO2 composite networks as ultralight and flexible supercapacitor electrodes. ACS Nano 2013, 7, 174–182. [Google Scholar] [CrossRef]
- Shanmugavani, A.; Selvan, R.K. Microwave assisted reflux synthesis of NiCo2O4/NiO composite: Fabrication of high performance asymmetric supercapacitor with Fe2O3. Electrochim. Acta 2016, 189, 283–294. [Google Scholar] [CrossRef]
- Jagadale, A.D.; Guan, G.; Li, X.; Du, X.; Ma, X.; Hao, X.; Abudula, A. Ultrathin nanoflakes of cobalt–manganese layered double hydroxide with high reversibility for asymmetric supercapacitor. J. Power Sources 2016, 306, 526–534. [Google Scholar] [CrossRef]
- Tang, C.; Tang, Z.; Gong, H. Hierarchically porous Ni-Co oxide for high reversibility asymmetric full-cell supercapacitors. J. Electrochem. Soc. 2012, 159, A651. [Google Scholar] [CrossRef]
- Li, M.; Luo, Y.; Jia, C.; Huang, M.; Yu, M.; Luo, G.; Zhao, L.; Boukherroub, R.; Jiang, Z. Au-assisted Polymerization of Conductive Poly (N-phenylglycine) as High-performance Positive Electrodes for Asymmetric Supercapacitors. Nanotechnology 2021, 33, 045602. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhao, K. Asymmetric flow electrochemical capacitor with high energy densities based on birnessite-type manganese oxide nanosheets and activated carbon slurries. J. Mater. Sci. 2016, 51, 9306–9313. [Google Scholar] [CrossRef]












| Performance Criteria | NCMSM//rGO SASC | NCMSM//rGO AASC |
|---|---|---|
| Working potential window | 1.4 V | 1.6 V |
| Specific capacitance/capacity (at 1 A g−1) | 51 F g−1/19.2 mAh g−1 | 58.3 F g−1/25.1 mAh g−1 |
| Maximum ED | 13.8 Wh kg−1 | 20.7 Wh kg−1 |
| Cycling stability (2000 cycles) | 93% | 96% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahoo, S.; Dhakal, G.; Kim, W.K.; Shim, J.-J. Ternary Nanohybrid of Ni3S2/CoMoS4/MnO2 on Nickel Foam for Aqueous and Solid-State High-Performance Supercapacitors. Nanomaterials 2022, 12, 1945. https://doi.org/10.3390/nano12111945
Sahoo S, Dhakal G, Kim WK, Shim J-J. Ternary Nanohybrid of Ni3S2/CoMoS4/MnO2 on Nickel Foam for Aqueous and Solid-State High-Performance Supercapacitors. Nanomaterials. 2022; 12(11):1945. https://doi.org/10.3390/nano12111945
Chicago/Turabian StyleSahoo, Sumanta, Ganesh Dhakal, Woo Kyoung Kim, and Jae-Jin Shim. 2022. "Ternary Nanohybrid of Ni3S2/CoMoS4/MnO2 on Nickel Foam for Aqueous and Solid-State High-Performance Supercapacitors" Nanomaterials 12, no. 11: 1945. https://doi.org/10.3390/nano12111945