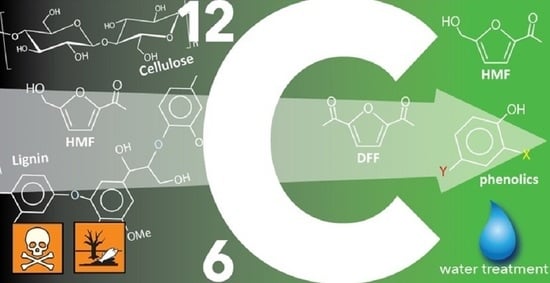
Carbon-Based Nanocatalysts (CnCs) for Biomass Valorization and Hazardous Organics Remediation
Abstract
:1. Introduction
2. Carbon-Based Nanocatalysts for the Production of 5-HMF from Cellulose and Starch-Rich Food Waste
2.1. Activated Carbon Based Catalysts and Derivatives
2.2. Carbon Nanotubes (CNTs) Based Catalysts
2.3. Graphene Based Catalysts
3. Photo- and Sono-Catalytic Selective Oxidation of HMF and Decomposition of Organic Pollutants
3.1. Carbon Nanotubes (CNTs) Based Catalysts
3.2. Carbon Quantum Dots (CQDs) Based Catalysts
3.3. Graphene-Based Nanocatalysts
3.4. Graphitic Carbon Nitride (g-C3N4) Based Nanocatalysts
3.5. Other Carbon-Based Materials
| Catalyst | Solvent | Experimental | HMF Conversion (%) | DFF Selectivity (%) | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Catalytic Loading (g/L) | Time (min) | Light Source | Concentration | |||||
| Bi2WO6/mpg–C3N4 | water | 10 | 8 | Vis. | 0.1 mM | 59 | 84 | [158] |
| g-C3N4/NaNbO3 | water | 10 | 8 | Vis. | 0.1 mmol | 35 | 87 | [157] |
| mesoporous carbon nitride | water | - | 48 | Vis. | 0.1 mmol | 38 | 99 | [152] |
| ultrathin graphitic carbon nitride | water | 1 | 5 | Vis. | 10 mM | 48 | 95 | [153] |
| Ni/CdS | water | 1 | 22 | Vis. | 10 mM | 22 | 100 | [178] |
| Au-Ru nanoparticles decorated reduced graphene oxides | toluene | 4 | 8 | Vis. | 0.5 mmol | 95.7 | 95 | [133] |
| WO3/g-C3N4 | ACN (3 mL) + PhCF3 (2 mL) | 10 | 6 | Vis. | 0.1 mmol | 27.4 | 87 | [156] |
| MXene/g-C3N4 composite (MX/CN) | benzotrifluoride | 10 | 10 | Vis. | 5 mM | 32 | 90 | [160] |
4. Lignin Hydrogenolysis to Valuable Phenolic Compounds
4.1. Activated Carbons
| Catalyst | Lignin | Reaction Conditions | Monomer Yield, % | Main Products | Ref. | ||
|---|---|---|---|---|---|---|---|
| Solvent | T (°C) | H2 (bar) | |||||
| 5%Cu/AC | Organosolv poplar | MeOH | 200 | 20 | 8.1 |  | [199] |
| 5%Ni/AC | 27.9 | ||||||
| 5%Ni- 5%Cu/AC | 40.2 | ||||||
| 5%Ni/AC | Biorefinery corncob | MeOH | 240 | 30 | 12.1 |  | [197] |
| EtOH | 8.4 | ||||||
| 5%Ru/AC | Enzymatic mild acidolysis | MeOH | 240 | 30 | 39.0 |  | [200] |
| 5%Pt/AC | Alkali | EtOH: H2O (65%) | 225 | 40 | 27.6 |  | [191] |
| 10%Ni/AC | Organosolv poplar | EtOH: IPA (1:1) | 270 | - | 58.0 |  | [198] |
| 10%Ni-5%Cu/AC | 63.4 | ||||||
| 10%Ni/AC | Organosolv | MeOH | 200 | 20 | 12.54 |  | [196] |
| 10%Fe/AC | 6.3 | ||||||
| 5%Ni-5%Fe/AC | 20.3 | ||||||
| 10%Pd/C | Enzymatic mild acidolysis | MeOH | 240 | 30 | 34.0 |  | [200] |
4.2. Graphene, N-doped and Graphene Nitride Type Carbons (g-C3N4)
4.3. Biomass Derived Carbons (Biochar, Lignin, etc.)
4.4. MOF-Derived Carbons
4.5. Carbides
4.6. Nano-Structured Carbons (Nanotubes, etc.)
5. Conclusions
- The design of CnCs with enhanced metal-support interaction and high dispersion of (nano)metals and oxides targeting to the development of efficient and stable catalysts in biomass transformation processes, that usually involve liquid (aqueous or solvent) phase reactions.
- Modification of CnCs by the addition of various surface functional groups can have a positive impact on the thermo/photo/sono-catalytic efficiency.
- CnCs catalyst can be effective in order to promote the in situ generation of hydrogen from hydrogen donor molecules, aiming to limit the external gas hydrogen.
- The development of ultrasound-assisted techniques for the advanced oxidation processes (AOPs) may decrease energy consumption leading to more sustainable methods.
- The formation of composite of different semiconductor-based materials with carbon-based supports leading to a red-shift of the active phases’ optical bandgap, elevating the CnCs’ ability to utilize better visible light to boost the catalytic activity.
- The synergistic coupling of sonochemistry with heterogeneous photocatalysis is a disruptive example within the process intensification concept either for synthesizing chemicals or/and decontaminating the water.
- Utilizing CnCs for continuous flow processes has barely been studied to date, hence it will be beneficial to do so towards more cost-effective processes that could be scaled-up for real-life applications.
Funding
Acknowledgments
Conflicts of Interest
References
- Cabana, L.; Ke, X.X.; Kepic, D.; Oro-Sole, J.; Tobias-Rossell, E.; Van Tendeloo, G.; Tobias, G. The role of steam treatment on the structure, purity and length distribution of multi-walled carbon nanotubes. Carbon 2015, 93, 1059–1067. [Google Scholar] [CrossRef]
- Jovanovic, S.P.; Syrgiannis, Z.; Markovic, Z.M.; Bonasera, A.; Kepic, D.P.; Budimir, M.D.; Milivojevic, D.D.; Spasojevic, V.D.; Dramicanin, M.D.; Pavlovic, V.B.; et al. Modification of Structural and Luminescence Properties of Graphene Quantum Dots by Gamma Irradiation and Their Application in a Photodynamic Therapy. ACS Appl. Mater. Interfaces 2015, 7, 25865–25874. [Google Scholar] [CrossRef] [PubMed]
- Kepic, D.; Markovic, Z.; Tosic, D.; Antunovic, I.H.; Adnadjevic, B.; Prekodravac, J.; Kleut, D.; Dramicanin, M.; Markovic, B.T. Surface modification of single-wall carbon nanotube thin films irradiated by microwaves: A Raman spectroscopy study. Phys. Scr. 2013, T157, 014040. [Google Scholar] [CrossRef]
- Mauter, M.S.; Elimelech, M. Environmental applications of carbon-based nanomaterials. Environ. Sci. Technol. 2008, 42, 5843–5859. [Google Scholar] [CrossRef]
- Notarianni, M.; Liu, J.; Vernon, K.; Motta, N. Synthesis and applications of carbon nanomaterials for energy generation and storage. Beilstein J. Nanotechnol. 2016, 7, 149–196. [Google Scholar] [CrossRef] [Green Version]
- Prekodravac, J.; Vasiljevic, B.; Markovic, Z.; Jovanovic, D.; Kleut, D.; Spitalsky, Z.; Micusik, M.; Danko, M.; Bajuk-Bogdanovic, D.; Todorovic-Markovic, B. Green and facile microwave assisted synthesis of (metal-free) N-doped carbon quantum dots for catalytic applications. Ceram. Int. 2019, 45, 17006–17013. [Google Scholar] [CrossRef]
- Prekodravac, J.R.; Kepic, D.P.; Colmenares, J.C.; Giannakoudakis, D.A.; Jovanovic, S.P. A comprehensive review on selected graphene synthesis methods: From electrochemical exfoliation through rapid thermal annealing towards biomass pyrolysis. J. Mater. Chem. C 2021, 9, 6722–6748. [Google Scholar] [CrossRef]
- Tosic, D.; Markovic, Z.; Jovanovic, S.; Prekodravac, J.; Budimir, M.; Kepic, D.; Holclajtner-Antunovic, I.; Dramicanin, M.; Todorovic-Markovic, B. Rapid thermal annealing of nickel-carbon nanowires for graphene nanoribbons formation. Synth. Met. 2016, 218, 43–49. [Google Scholar] [CrossRef]
- Cooper, A.I.; Bojdys, M.J. Carbon nitride vs. graphene—Now in 2D! Mater. Today 2014, 17, 468–469. [Google Scholar] [CrossRef]
- Pham, V.P.; Jang, H.S.; Whang, D.; Choi, J.Y. Direct growth of graphene on rigid and flexible substrates: Progress, applications, and challenges. Chem. Soc. Rev. 2017, 46, 6276–6300. [Google Scholar] [CrossRef]
- Allen, S.J.; Whitten, L.; McKay, G. The Production and Characterisation of Activated Carbons: A Review. Dev. Chem. Eng. Miner. Process. 2008, 6, 231–261. [Google Scholar] [CrossRef]
- Ania, C.O.; Armstrong, P.A.; Bandosz, T.J.; Beguin, F.; Carvalho, A.P.; Celzard, A.; Frackowiak, E.; Gilarranz, M.A.; László, K.; Matos, J.; et al. Engaging nanoporous carbons in “beyond adsorption” applications: Characterization, challenges and performance. Carbon 2020, 164, 69–84. [Google Scholar] [CrossRef]
- Bandosz, T.J. Exploring the Silent Aspect of Carbon Nanopores. Nanomaterials 2021, 11, 407. [Google Scholar] [CrossRef]
- Dabrowski, A.; Podkoscielny, P.; Hubicki, Z.; Barczak, M. Adsorption of phenolic compounds by activated carbon--a critical review. Chemosphere 2005, 58, 1049–1070. [Google Scholar] [CrossRef] [PubMed]
- Bedia, J.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodriguez, J.J.; Belver, C. A Review on the Synthesis and Characterization of Biomass-Derived Carbons for Adsorption of Emerging Contaminants from Water. C—J. Carbon Res. 2018, 4, 63. [Google Scholar] [CrossRef] [Green Version]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Gao, Y.; Yue, Q.; Gao, B.; Li, A. Insight into activated carbon from different kinds of chemical activating agents: A review. Sci. Total Environ. 2020, 746, 141094. [Google Scholar] [CrossRef]
- Srivastava, A.; Gupta, B.; Majumder, A.; Gupta, A.K.; Nimbhorkar, S.K. A comprehensive review on the synthesis, performance, modifications, and regeneration of activated carbon for the adsorptive removal of various water pollutants. J. Environ. Chem. Eng. 2021, 9, 106177. [Google Scholar] [CrossRef]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Hosseini-Bandegharaei, A.; Tsafrakidou, P.; Triantafyllidis, K.S.; Kornaros, M.; Anastopoulos, I. Aloe vera waste biomass-based adsorbents for the removal of aquatic pollutants: A review. J. Environ. Manag. 2018, 227, 354–364. [Google Scholar] [CrossRef]
- Madhubashani, A.M.P.; Giannakoudakis, D.A.; Amarasinghe, B.; Rajapaksha, A.U.; Pradeep Kumara, P.B.T.; Triantafyllidis, K.S.; Vithanage, M. Propensity and appraisal of biochar performance in removal of oil spills: A comprehensive review. Environ. Pollut. 2021, 288, 117676. [Google Scholar] [CrossRef] [PubMed]
- Titirici, M.M.; Antonietti, M.; Baccile, N. Hydrothermal carbon from biomass: A comparison of the local structure from poly- to monosaccharides and pentoses/hexoses. Green Chem. 2008, 10, 1204–1212. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.H.; Shen, D.K.; Wu, C.F.; Gu, S. State-of-the-art on the production and application of carbon nanomaterials from biomass. Green Chem. 2018, 20, 5031–5057. [Google Scholar] [CrossRef] [Green Version]
- Delbecq, F.; Len, C. Recent Advances in the Microwave-Assisted Production of Hydroxymethylfurfural by Hydrolysis of Cellulose Derivatives-A Review. Molecules 2018, 23, 1973. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Verrier, C.; Queneau, Y.; Popowycz, F. 5-Hydroxymethylfurfural (HMF) in Organic Synthesis: A Review of its Recent Applications Towards Fine Chemicals. Curr. Org. Synth. 2019, 16, 583–614. [Google Scholar] [CrossRef]
- Su, T.; Zhao, D.; Wang, Y.; Lu, H.; Varma, R.S.; Len, C. Innovative Protocols in the Catalytic Oxidation of 5-Hydroxymethylfurfural. ChemSusChem 2021, 14, 266–280. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Su, T.; Wang, Y.T.; Varma, R.S.; Len, C. Recent advances in catalytic oxidation of 5-hydroxymethylfurfural. Mol. Catal. 2020, 495, 111133. [Google Scholar] [CrossRef]
- da Costa, N.L.; Pereira, L.G.; Resende, J.V.M.; Mendoza, C.A.D.; Ferreira, K.K.; Detoni, C.; Souza, M.M.V.M.; Gomes, F.N.D.C. Phosphotungstic acid on activated carbon: A remarkable catalyst for 5-hydroxymethylfurfural production. Mol. Catal. 2021, 500, 111334. [Google Scholar] [CrossRef]
- Wang, J.J.; Xu, W.J.; Ren, J.W.; Liu, X.H.; Lu, G.Z.; Wang, Y.Q. Efficient catalytic conversion of fructose into hydroxymethylfurfural by a novel carbon-based solid acid. Green Chem. 2011, 13, 2678–2681. [Google Scholar] [CrossRef]
- Rusanen, A.; Lahti, R.; Lappalainen, K.; Karkkainen, J.; Hu, T.; Romar, H.; Lassi, U. Catalytic conversion of glucose to 5-hydroxymethylfurfural over biomass-based activated carbon catalyst. Catal. Today 2020, 357, 94–101. [Google Scholar] [CrossRef]
- Ji, H.P.; Fu, J.; Wang, T.F. Pyrolyzing Renewable Sugar and Taurine on the Surface of Multi-Walled Carbon Nanotubes as Heterogeneous Catalysts for Hydroxymethylfurfural Production. Catalysts 2018, 8, 517. [Google Scholar] [CrossRef] [Green Version]
- Ji, T.; Tu, R.; Mu, L.; Lu, X.; Zhu, J. Enhancing Energy Efficiency in Saccharide–HMF Conversion with Core/shell Structured Microwave Responsive Catalysts. ACS Sustain. Chem. Eng. 2017, 5, 4352–4358. [Google Scholar] [CrossRef]
- Kumar, S.; Gawande, M.B.; Kopp, J.; Kment, S.; Varma, R.S.; Zboril, R. P- and F-co-doped Carbon Nitride Nanocatalysts for Photocatalytic CO2 Reduction and Thermocatalytic Furanics Synthesis from Sugars. ChemSusChem 2020, 13, 5231–5238. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Baig, R.B.N.; Nadagouda, M.N.; Len, C.; Varma, R.S. Sustainable pathway to furanics from biomass via heterogeneous organo-catalysis. Green Chem. 2017, 19, 164–168. [Google Scholar] [CrossRef]
- Hirano, Y.; Beltramini, J.N.; Mori, A.; Nakamura, M.; Karim, M.R.; Kim, Y.; Nakamura, M.; Hayami, S. Microwave-assisted catalytic conversion of glucose to 5-hydroxymethylfurfural using “three dimensional” graphene oxide hybrid catalysts. RSC Adv. 2020, 10, 11727–11736. [Google Scholar] [CrossRef]
- Shaikh, M.; Singh, S.K.; Khilari, S.; Sahu, M.; Ranganath, K.V.S. Graphene oxide as a sustainable metal and solvent free catalyst for dehydration of fructose to 5-HMF: A new and green protocol. Catal. Commun. 2018, 106, 64–67. [Google Scholar] [CrossRef]
- Azar, F.Z.; Lillo-Rodenas, M.A.; Roman-Martinez, M.C. Cellulose hydrolysis catalysed by mesoporous activated carbons functionalized under mild conditions. SN Appl. Sci. 2019, 1, 1739. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Yu, I.K.M.; Chen, S.S.; Tsang, D.C.W.; Wang, L.; Xiong, X.; Zhang, S.; Ok, Y.S.; Kwon, E.E.; Song, H.; et al. Production of 5-hydroxymethylfurfural from starch-rich food waste catalyzed by sulfonated biochar. Bioresour. Technol. 2018, 252, 76–82. [Google Scholar] [CrossRef]
- Delbecq, F.; Wang, Y.T.; Len, C. Various carbohydrate precursors dehydration to 5-HMF in an acidic biphasic system under microwave heating using betaine as a co-catalyst. Mol. Catal. 2017, 434, 80–85. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.M.; Tsang, D.C.W.; Zhang, S.; Ok, Y.S.; Kwon, E.E.; Song, H.; Poon, C.S. Phosphoric acid-activated wood biochar for catalytic conversion of starch-rich food waste into glucose and 5-hydroxymethylfurfural. Bioresour. Technol. 2018, 267, 242–248. [Google Scholar] [CrossRef]
- Tyagi, U.; Anand, N.; Kumar, D. Synergistic effect of modified activated carbon and ionic liquid in the conversion of microcrystalline cellulose to 5-Hydroxymethyl Furfural. Bioresour. Technol. 2018, 267, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Bado-Nilles, A.; Diallo, A.O.; Marlair, G.; Pandard, P.; Chabot, L.; Geffard, A.; Len, C.; Porcher, J.M.; Sanchez, W. Coupling of OECD standardized test and immunomarkers to select the most environmentally benign ionic liquids option--towards an innovative "safety by design" approach. J. Hazard. Mater. 2015, 283, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Diallo, A.O.; Fayet, G.; Len, C.; Marlair, G. Evaluation of Heats of Combustion of Ionic Liquids through Use of Existing and Purpose-Built Models. Ind. Eng. Chem. Res. 2012, 51, 3149–3156. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, Z.T.; Fu, Z.H.; Liu, Y.C.; Yi, X.F.; Zheng, A.M.; Kirk, S.R.; Yin, D.L. Effective transformation of cellulose to 5-hydroxymethylfurfural catalyzed by fluorine anion-containing ionic liquid modified biochar sulfonic acids in water. Cellulose 2017, 24, 95–106. [Google Scholar] [CrossRef]
- Liu, L.; Yang, X.; Hou, Q.D.; Zhang, S.Q.; Ju, M.T. Corn stalk conversion into 5-hydroxymethylfurfural by modified biochar catalysis in a multi-functional solvent. J. Clean. Prod. 2018, 187, 380–389. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W.; Yip, A.C.K.; Chen, S.S.; Wang, L.; Ok, Y.S.; Poon, C.S. Catalytic valorization of starch-rich food waste into hydroxymethylfurfural (HMF): Controlling relative kinetics for high productivity. Bioresour. Technol. 2017, 237, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Khan, D.; Ali, Z.; Asif, D.; Kumar Panjwani, M.; Khan, I. Incorporation of carbon nanotubes in photoactive layer of organic solar cells. Ain Shams Eng. J. 2021, 12, 897–900. [Google Scholar] [CrossRef]
- Faba, L.; Garces, D.; Diaz, E.; Ordonez, S. Carbon Materials as Phase-Transfer Promoters for Obtaining 5-Hydroxymethylfurfural from Cellulose in a Biphasic System. ChemSusChem 2019, 12, 3769–3777. [Google Scholar] [CrossRef]
- Wallace, P.R. The Band Theory of Graphite. Phys. Rev. 1947, 71, 622–634. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-F.; Liu, Y.-G.; Zeng, G.-M.; Liu, N.; Liu, S.-B. Graphene and graphene-based nanocomposites used for antibiotics removal in water treatment: A review. Chemosphere 2019, 226, 360–380. [Google Scholar] [CrossRef] [PubMed]
- Jilani, A.; Othman, M.H.D.; Ansari, M.O.; Hussain, S.Z.; Ismail, A.F.; Khan, I.U. Graphene and its derivatives: Synthesis, modifications, and applications in wastewater treatment. Environ. Chem. Lett. 2018, 16, 1301–1323. [Google Scholar] [CrossRef]
- Szabó, T.; Berkesi, O.; Forgó, P.; Josepovits, K.; Sanakis, Y.; Petridis, D.; Dékány, I. Evolution of Surface Functional Groups in a Series of Progressively Oxidized Graphite Oxides. Chem. Mater. 2006, 18, 2740–2749. [Google Scholar] [CrossRef]
- Saroyan, H.S.; Bele, S.; Giannakoudakis, D.A.; Samanidou, V.F.; Bandosz, T.J.; Deliyanni, E.A. Degradation of endocrine disruptor, bisphenol-A, on an mixed oxidation state manganese oxide/modified graphite oxide composite: A role of carbonaceous phase. J. Colloid Interface Sci. 2019, 539, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Muthoosamy, K.; Manickam, S. State of the art and recent advances in the ultrasound-assisted synthesis, exfoliation and functionalization of graphene derivatives. Ultrason. Sonochem. 2017, 39, 478–493. [Google Scholar] [CrossRef]
- Soltani, T.; Kyu Lee, B. A benign ultrasonic route to reduced graphene oxide from pristine graphite. J. Colloid Interface Sci. 2017, 486, 337–343. [Google Scholar] [CrossRef]
- Li, K.X.; Chen, J.; Yan, Y.B.; Min, Y.G.; Li, H.P.; Xi, F.N.; Liu, J.Y.; Chen, P. Quasi-homogeneous carbocatalysis for one-pot selective conversion of carbohydrates to 5-hydroxymethylfurfural using sulfonated graphene quantum dots. Carbon 2018, 136, 224–233. [Google Scholar] [CrossRef]
- Petrier, C.; Jiang, Y.; Lamy, M.-F. Ultrasound and Environment: Sonochemical Destruction of Chloroaromatic Derivatives. Environ. Sci. Technol. 1998, 32, 1316–1318. [Google Scholar] [CrossRef]
- Chatel, G.; Valange, S.; Behling, R.; Colmenares, J.C. A Combined Approach using Sonochemistry and Photocatalysis: How to Apply Sonophotocatalysis for Biomass Conversion? ChemCatChem 2017, 9, 2615–2621. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Łomot, D.; Colmenares, J.C. When sonochemistry meets heterogeneous photocatalysis: Designing a sonophotoreactor towards sustainable selective oxidation. Green Chem. 2020, 22, 4896–4905. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Luque, R. Heterogeneous photocatalytic nanomaterials: Prospects and challenges in selective transformations of biomass-derived compounds. Chem. Soc. Rev. 2014, 43, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Chatel, G.; Colmenares, J.C. Sonochemistry: From Basic Principles to Innovative Applications. Top. Curr. Chem. 2017, 375, 8. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamadani, Y.A.J.; Jung, C.; Im, J.-K.; Boateng, L.K.; Flora, J.R.V.; Jang, M.; Heo, J.; Park, C.M.; Yoon, Y. Sonocatalytic degradation coupled with single-walled carbon nanotubes for removal of ibuprofen and sulfamethoxazole. Chem. Eng. Sci. 2017, 162, 300–308. [Google Scholar] [CrossRef]
- Li, S.; Wang, G.; Qiao, J.; Zhou, Y.; Ma, X.; Zhang, H.; Li, G.; Wang, J.; Song, Y. Sonocatalytic degradation of norfloxacin in aqueous solution caused by a novel Z-scheme sonocatalyst, mMBIP-MWCNT-In2O3 composite. J. Mol. Liq. 2018, 254, 166–176. [Google Scholar] [CrossRef]
- Panahian, Y.; Arsalani, N. Synthesis of Hedgehoglike F-TiO2(B)/CNT Nanocomposites for Sonophotocatalytic and Photocatalytic Degradation of Malachite Green (MG) under Visible Light: Kinetic Study. J. Phys. Chem. A 2017, 121, 5614–5624. [Google Scholar] [CrossRef]
- Wang, S.; Gong, Q.; Liang, J. Sonophotocatalytic degradation of methyl orange by carbon nanotube/TiO2 in aqueous solutions. Ultrason. Sonochem. 2009, 16, 205–208. [Google Scholar] [CrossRef]
- Reheman, A.; Kadeer, K.; Okitsu, K.; Halidan, M.; Tursun, Y.; Dilinuer, T.; Abulikemu, A. Facile photo-ultrasonic assisted reduction for preparation of rGO/Ag2CO3 nanocomposites with enhanced photocatalytic oxidation activity for tetracycline. Ultrason. Sonochem. 2019, 51, 166–177. [Google Scholar] [CrossRef]
- Vinesh, V.; Shaheer, A.R.M.; Neppolian, B. Reduced graphene oxide (rGO) supported electron deficient B-doped TiO2 (Au/B-TiO2/rGO) nanocomposite: An efficient visible light sonophotocatalyst for the degradation of Tetracycline (TC). Ultrason. Sonochem. 2019, 50, 302–310. [Google Scholar] [CrossRef]
- Khataee, A.; Sadeghi Rad, T.; Nikzat, S.; Hassani, A.; Aslan, M.H.; Kobya, M.; Demirbaş, E. Fabrication of NiFe layered double hydroxide/reduced graphene oxide (NiFe-LDH/rGO) nanocomposite with enhanced sonophotocatalytic activity for the degradation of moxifloxacin. Chem. Eng. J. 2019, 375, 122102. [Google Scholar] [CrossRef]
- Sadeghi Rad, T.; Khataee, A.; Arefi-Oskoui, S.; Sadeghi Rad, S.; Orooji, Y.; Gengec, E.; Kobya, M. Graphene-based ZnCr layered double hydroxide nanocomposites as bactericidal agents with high sonophotocatalytic performances for degradation of rifampicin. Chemosphere 2022, 286, 131740. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi Rad, T.; Khataee, A.; Sadeghi Rad, S.; Arefi-Oskoui, S.; Gengec, E.; Kobya, M.; Yoon, Y. Zinc-chromium layered double hydroxides anchored on carbon nanotube and biochar for ultrasound-assisted photocatalysis of rifampicin. Ultrason. Sonochem. 2022, 82, 105875. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, E.A.N.; Cividanes, L.d.S.; Fonseca, B.C.d.S.; de Freitas, A.P.B.R.; Coutinho, A.d.R.; Thim, G.P. TiO2—Carbon composite using coconut waste as carbon source: Sonocatalysis and adsorption evaluation. Surf. Interfaces 2018, 12, 124–134. [Google Scholar] [CrossRef]
- Kakavandi, B.; Bahari, N.; Rezaei Kalantary, R.; Dehghani Fard, E. Enhanced sono-photocatalysis of tetracycline antibiotic using TiO2 decorated on magnetic activated carbon (MAC@T) coupled with US and UV: A new hybrid system. Ultrason. Sonochem. 2019, 55, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Khataee, A.; Kayan, B.; Gholami, P.; Kalderis, D.; Akay, S. Sonocatalytic degradation of an anthraquinone dye using TiO2-biochar nanocomposite. Ultrason. Sonochem. 2017, 39, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M.; Ashraf, A.M.; Abukhadra, M.R. TiO2 Nanoribbons/Carbon Nanotubes Composite with Enhanced Photocatalytic Activity; Fabrication, Characterization, and Application. Sci. Rep. 2018, 8, 781. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Qian, J.; Wang, N.; Xing, J.; Liu, L. In-situ synthesis of CNT/TiO2 heterojunction nanocomposite and its efficient photocatalytic degradation of Rhodamine B dye. Inorg. Chem. Commun. 2020, 119, 108071. [Google Scholar] [CrossRef]
- Phin, H.-Y.; Ong, Y.-T.; Sin, J.-C. Effect of carbon nanotubes loading on the photocatalytic activity of zinc oxide/carbon nanotubes photocatalyst synthesized via a modified sol-gel method. J. Environ. Chem. Eng. 2020, 8, 103222. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, E.; Hong, Z.L.; Ahmed, W.; Elhissi, A.; Khalid, N.R. Photocatalytic, sonocatalytic and sonophotocatalytic degradation of Rhodamine B using ZnO/CNTs composites photocatalysts. Ultrason. Sonochem. 2014, 21, 761–773. [Google Scholar] [CrossRef]
- Zhou, C.; Deng, W.; Wan, X.; Zhang, Q.; Yang, Y.; Wang, Y. Functionalized Carbon Nanotubes for Biomass Conversion: The Base-Free Aerobic Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid over Platinum Supported on a Carbon Nanotube Catalyst. ChemCatChem 2015, 7, 2853–2863. [Google Scholar] [CrossRef]
- Travlou, N.A.; Giannakoudakis, D.A.; Algarra, M.; Labella, A.M.; Rodríguez-Castellón, E.; Bandosz, T.J. S- and N-doped carbon quantum dots: Surface chemistry dependent antibacterial activity. Carbon 2018, 135, 104–111. [Google Scholar] [CrossRef]
- Algarra, M.; Pérez-Martín, M.; Cifuentes-Rueda, M.; Jiménez-Jiménez, J.; Esteves da Silva, J.C.G.; Bandosz, T.J.; Rodríguez-Castellón, E.; López Navarrete, J.T.; Casado, J. Carbon dots obtained using hydrothermal treatment of formaldehyde. Cell imaging in vitro. Nanoscale 2014, 6, 9071–9077. [Google Scholar] [CrossRef] [PubMed]
- Travlou, N.A.; Secor, J.; Bandosz, T.J. Highly luminescent S-doped carbon dots for the selective detection of ammonia. Carbon 2017, 114, 544–556. [Google Scholar] [CrossRef]
- Das, R.; Bandyopadhyay, R.; Pramanik, P. Carbon quantum dots from natural resource: A review. Mater. Today Chem. 2018, 8, 96–109. [Google Scholar] [CrossRef]
- Rodríguez-Padrón, D.; Algarra, M.; Tarelho, L.A.C.; Frade, J.; Franco, A.; de Miguel, G.; Jiménez, J.; Rodríguez-Castellón, E.; Luque, R. Catalyzed Microwave-Assisted Preparation of Carbon Quantum Dots from Lignocellulosic Residues. ACS Sustain. Chem. Eng. 2018, 6, 7200–7205. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Asadi, A.; Sillanpää, M.; Farhadian, N. Application of carbon quantum dots to increase the activity of conventional photocatalysts: A systematic review. J. Mol. Liq. 2018, 271, 857–871. [Google Scholar] [CrossRef]
- Wang, R.; Lu, K.-Q.; Tang, Z.-R.; Xu, Y.-J. Recent progress in carbon quantum dots: Synthesis, properties and applications in photocatalysis. J. Mater. Chem. A 2017, 5, 3717–3734. [Google Scholar] [CrossRef]
- Hu, S.; Tian, R.; Wu, L.; Zhao, Q.; Yang, J.; Liu, J.; Cao, S. Chemical Regulation of Carbon Quantum Dots from Synthesis to Photocatalytic Activity. Chem. Asian J. 2013, 8, 1035–1041. [Google Scholar] [CrossRef]
- Long, C.; Jiang, Z.; Shangguan, J.; Qing, T.; Zhang, P.; Feng, B. Applications of carbon dots in environmental pollution control: A review. Chem. Eng. J. 2021, 406, 126848. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, H.; Zhao, J.; Jiang, D.; Zhan, Q. Carbon quantum dot (CQD)-modified Bi3O4Br nanosheets possessing excellent photocatalytic activity under simulated sunlight. Mater. Sci. Semicond. Process. 2021, 122, 105489. [Google Scholar] [CrossRef]
- Hazarika, D.; Karak, N. Photocatalytic degradation of organic contaminants under solar light using carbon dot/titanium dioxide nanohybrid, obtained through a facile approach. Appl. Surf. Sci. 2016, 376, 276–285. [Google Scholar] [CrossRef]
- Shen, T.; Wang, Q.; Guo, Z.; Kuang, J.; Cao, W. Hydrothermal synthesis of carbon quantum dots using different precursors and their combination with TiO2 for enhanced photocatalytic activity. Ceram. Int. 2018, 44, 11828–11834. [Google Scholar] [CrossRef]
- Miao, R.; Luo, Z.; Zhong, W.; Chen, S.-Y.; Jiang, T.; Dutta, B.; Nasr, Y.; Zhang, Y.; Suib, S.L. Mesoporous TiO2 modified with carbon quantum dots as a high-performance visible light photocatalyst. Appl. Catal. B Environ. 2016, 189, 26–38. [Google Scholar] [CrossRef] [Green Version]
- Olmos-Moya, P.M.; Velazquez-Martinez, S.; Pineda-Arellano, C.; Rangel-Mendez, J.R.; Chazaro-Ruiz, L.F. High added value functionalized carbon quantum dots synthetized from orange peels by assisted microwave solvothermal method and their performance as photosensitizer of mesoporous TiO2 photoelectrodes. Carbon 2022, 187, 216–229. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Q.; Wang, J.; He, H.; Shi, F.; Xing, B.; Jia, J.; Huang, G.; Zhang, C. Facile preparation of carbon quantum dots/TiO2 composites at room temperature with improved visible-light photocatalytic activity. J. Alloys Compd. 2021, 869, 159389. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.-P.; Zhao, J.-X.; Ge, Z.-H.; Zhao, X.-K.; Zou, L. ZnO/carbon quantum dots heterostructure with enhanced photocatalytic properties. Appl. Surf. Sci. 2013, 279, 367–373. [Google Scholar] [CrossRef]
- Sharma, S.; Mehta, S.K.; Kansal, S.K. N doped ZnO/C-dots nanoflowers as visible light driven photocatalyst for the degradation of malachite green dye in aqueous phase. J. Alloys Compd. 2017, 699, 323–333. [Google Scholar] [CrossRef]
- Muthulingam, S.; Lee, I.-H.; Uthirakumar, P. Highly efficient degradation of dyes by carbon quantum dots/N-doped zinc oxide (CQD/N-ZnO) photocatalyst and its compatibility on three different commercial dyes under daylight. J. Colloid Interface Sci. 2015, 455, 101–109. [Google Scholar] [CrossRef]
- Bonet-San-Emeterio, M.; Algarra, M.; Petković, M.; del Valle, M. Modification of electrodes with N-and S-doped carbon dots. Evaluation of the electrochemical response. Talanta 2020, 212, 120806. [Google Scholar] [CrossRef]
- Marković, Z.M.; Labudová, M.; Danko, M.; Matijašević, D.; Mičušík, M.; Nádaždy, V.; Kováčová, M.; Kleinová, A.; Špitalský, Z.; Pavlović, V.; et al. Highly Efficient Antioxidant F- and Cl-Doped Carbon Quantum Dots for Bioimaging. ACS Sustain. Chem. Eng. 2020, 8, 16327–16338. [Google Scholar] [CrossRef]
- Louleb, M.; Latrous, L.; Ríos, Á.; Zougagh, M.; Rodríguez-Castellón, E.; Algarra, M.; Soto, J. Detection of Dopamine in Human Fluids Using N-Doped Carbon Dots. ACS Appl. Nano Mater. 2020, 3, 8004–8011. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, G.; Liang, X.; Dong, X.; Zhang, X. Supporting carbon quantum dots on NH2-MIL-125 for enhanced photocatalytic degradation of organic pollutants under a broad spectrum irradiation. Appl. Surf. Sci. 2019, 467–468, 320–327. [Google Scholar] [CrossRef]
- Al-Hamadani, Y.A.J.; Lee, G.; Kim, S.; Park, C.M.; Jang, M.; Her, N.; Han, J.; Kim, D.-H.; Yoon, Y. Sonocatalytic degradation of carbamazepine and diclofenac in the presence of graphene oxides in aqueous solution. Chemosphere 2018, 205, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Putri, L.K.; Ong, W.-J.; Chang, W.S.; Chai, S.-P. Heteroatom doped graphene in photocatalysis: A review. Appl. Surf. Sci. 2015, 358, 2–14. [Google Scholar] [CrossRef]
- Faraldos, M.; Bahamonde, A. Environmental applications of titania-graphene photocatalysts. Catal. Today 2017, 285, 13–28. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-Graphene Composite as a High Performance Photocatalyst. ACS Nano 2010, 4, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Szabó, T.; Veres, Á.; Cho, E.; Khim, J.; Varga, N.; Dékány, I. Photocatalyst separation from aqueous dispersion using graphene oxide/TiO2 nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2013, 433, 230–239. [Google Scholar] [CrossRef]
- Minella, M.; Sordello, F.; Minero, C. Photocatalytic process in TiO2/graphene hybrid materials. Evidence of charge separation by electron transfer from reduced graphene oxide to TiO2. Catal. Today 2017, 281, 29–37. [Google Scholar] [CrossRef]
- Aleksandrzak, M.; Adamski, P.; Kukułka, W.; Zielinska, B.; Mijowska, E. Effect of graphene thickness on photocatalytic activity of TiO2-graphene nanocomposites. Appl. Surf. Sci. 2015, 331, 193–199. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Farahmand, N.; Łomot, D.; Sobczak, K.; Bandosz, T.J.; Colmenares, J.C. Ultrasound-activated TiO2/GO-based bifunctional photoreactive adsorbents for detoxification of chemical warfare agent surrogate vapors. Chem. Eng. J. 2020, 395, 125099. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Vikrant, K.; LaGrow, A.P.; Lisovytskiy, D.; Kim, K.-H.; Bandosz, T.J.; Carlos Colmenares, J. Scrolled titanate nanosheet composites with reduced graphite oxide for photocatalytic and adsorptive removal of toxic vapors. Chem. Eng. J. 2021, 415, 128907. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Anju, S.M. Synthesis and evaluation of TiO2 nanotubes/silylated graphene oxide-based molecularly imprinted polymer for the selective adsorption and subsequent photocatalytic degradation of 2,4-Dichlorophenoxyacetic acid. J. Environ. Chem. Eng. 2019, 7, 103355. [Google Scholar] [CrossRef]
- Mei, J.-Y.; Qi, P.; Wei, X.-N.; Zheng, X.-C.; Wang, Q.; Guan, X.-X. Assembly and enhanced elimination performance of 3D graphene aerogel-zinc oxide hybrids for methylene blue dye in water. Mater. Res. Bull. 2019, 109, 141–148. [Google Scholar] [CrossRef]
- Kheirabadi, M.; Samadi, M.; Asadian, E.; Zhou, Y.; Dong, C.; Zhang, J.; Moshfegh, A.Z. Well-designed Ag/ZnO/3D graphene structure for dye removal: Adsorption, photocatalysis and physical separation capabilities. J. Colloid Interface Sci. 2019, 537, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhu, Y.; Zhu, G.; Zhang, Z.; Chen, X.; Yao, W. Three-dimensional photocatalysts with a network structure. J. Mater. Chem. A 2017, 5, 5661–5679. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Bandosz, T.J. Building MOF Nanocomposites with Oxidized Graphitic Carbon Nitride Nanospheres: The Effect of Framework Geometry on the Structural Heterogeneity. Molecules 2019, 24, 4529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannakoudakis, D.A.; Bandosz, T.J. Defectous UiO-66 MOF Nanocomposites as Reactive Media of Superior Protection against Toxic Vapors. ACS Appl. Mater. Interfaces 2020, 12, 14678–14689. [Google Scholar] [CrossRef]
- Cai, J.; Liu, W.; Li, Z. One-pot self-assembly of Cu2O/RGO composite aerogel for aqueous photocatalysis. Appl. Surf. Sci. 2015, 358, 146–151. [Google Scholar] [CrossRef]
- Pham, T.-T.; Nguyen-Huy, C.; Shin, E.W. NiTiO3/reduced graphene oxide materials synthesized by a two-step microwave-assisted method. Mater. Lett. 2016, 184, 38–42. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Soin, N.; Roy, S.S. Role of graphene/metal oxide composites as photocatalysts, adsorbents and disinfectants in water treatment: A review. RSC Adv. 2014, 4, 3823–3851. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Petit, C. MOF/graphite oxide hybrid materials: Exploring the new concept of adsorbents and catalysts. Adsorption 2011, 17, 5–16. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, P.; Zhou, H.-C.; Sharma, V.K. Water-stable metal-organic frameworks for aqueous removal of heavy metals and radionuclides: A review. Chemosphere 2018, 209, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Manousi, N.; Giannakoudakis, D.A.; Rosenberg, E.; Zachariadis, G.A. Extraction of Metal Ions with Metal–Organic Frameworks. Molecules 2019, 24, 4605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Liu, B. Synthesis of a novel and stable reduced graphene oxide/MOF hybrid nanocomposite and photocatalytic performance for the degradation of dyes. RSC Adv. 2016, 6, 17873–17879. [Google Scholar] [CrossRef]
- Thi, Q.V.; Tamboli, M.S.; Thanh Hoai Ta, Q.; Kolekar, G.B.; Sohn, D. A nanostructured MOF/reduced graphene oxide hybrid for enhanced photocatalytic efficiency under solar light. Mater. Sci. Eng. B 2020, 261, 114678. [Google Scholar] [CrossRef]
- Yang, C.; You, X.; Cheng, J.; Zheng, H.; Chen, Y. A novel visible-light-driven In-based MOF/graphene oxide composite photocatalyst with enhanced photocatalytic activity toward the degradation of amoxicillin. Appl. Catal. B Environ. 2017, 200, 673–680. [Google Scholar] [CrossRef]
- El-Fawal, E.M.; Younis, S.A.; Zaki, T. Designing AgFeO2-graphene/Cu2(BTC)3 MOF heterojunction photocatalysts for enhanced treatment of pharmaceutical wastewater under sunlight. J. Photochem. Photobiol. A Chem. 2020, 401, 112746. [Google Scholar] [CrossRef]
- Chen, Y.; Zhai, B.; Liang, Y.; Li, Y. Hybrid photocatalysts using semiconductor/MOF/graphene oxide for superior photodegradation of organic pollutants under visible light. Mater. Sci. Semicond. Process. 2020, 107, 104838. [Google Scholar] [CrossRef]
- Chen, Y.; Zhai, B.; Liang, Y. Enhanced degradation performance of organic dyes removal by semiconductor/MOF/graphene oxide composites under visible light irradiation. Diam. Relat. Mater. 2019, 98, 107508. [Google Scholar] [CrossRef]
- Babu, S.G.; Karthik, P.; John, M.C.; Lakhera, S.K.; Ashokkumar, M.; Khim, J.; Neppolian, B. Synergistic effect of sono-photocatalytic process for the degradation of organic pollutants using CuO-TiO2/rGO. Ultrason. Sonochem. 2019, 50, 218–223. [Google Scholar] [CrossRef]
- Khairy, M.; Naguib, E.M.; Mohamed, M.M. Enhancement of Photocatalytic and Sonophotocatalytic Degradation of 4-nitrophenol by ZnO/Graphene Oxide and ZnO/Carbon Nanotube Nanocomposites. J. Photochem. Photobiol. A Chem. 2020, 396, 112507. [Google Scholar] [CrossRef]
- Nirumand, L.; Farhadi, S.; Zabardasti, A.; Khataee, A. Copper ferrite nanoparticles supported on MIL-101/reduced graphene oxide as an efficient and recyclable sonocatalyst. J. Taiwan Inst. Chem. Eng. 2018, 93, 674–685. [Google Scholar] [CrossRef]
- Ma, B.; Wang, Y.; Guo, X.; Tong, X.; Liu, C.; Wang, Y.; Guo, X. Photocatalytic synthesis of 2,5-diformylfuran from 5-hydroxymethyfurfural or fructose over bimetallic Au-Ru nanoparticles supported on reduced graphene oxides. Appl. Catal. A Gen. 2018, 552, 70–76. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Ismael, M.; Wu, Y. A mini-review on the synthesis and structural modification of g-C3N4-based materials, and their applications in solar energy conversion and environmental remediation. Sustain. Energy Fuels 2019, 3, 2907–2925. [Google Scholar] [CrossRef]
- Akhundi, A.; Badiei, A.; Ziarani, G.M.; Habibi-Yangjeh, A.; Muñoz-Batista, M.J.; Luque, R. Graphitic carbon nitride-based photocatalysts: Toward efficient organic transformation for value-added chemicals production. Mol. Catal. 2020, 488, 110902. [Google Scholar] [CrossRef]
- de Almeida Ribeiro, R.S.; Monteiro Ferreira, L.E.; Rossa, V.; Lima, C.G.S.; Paixão, M.W.; Varma, R.S.; de Melo Lima, T. Graphitic Carbon Nitride-Based Materials as Catalysts for the Upgrading of Lignocellulosic Biomass-Derived Molecules. ChemSusChem 2020, 13, 3992–4004. [Google Scholar] [CrossRef]
- Wang, L.; Wang, K.; He, T.; Zhao, Y.; Song, H.; Wang, H. Graphitic Carbon Nitride-Based Photocatalytic Materials: Preparation Strategy and Application. ACS Sustain. Chem. Eng. 2020, 8, 16048–16085. [Google Scholar] [CrossRef]
- Gong, Y.; Li, M.; Wang, Y. Carbon Nitride in Energy Conversion and Storage: Recent Advances and Future Prospects. ChemSusChem 2015, 8, 931–946. [Google Scholar] [CrossRef]
- Xu, W.; Lai, S.; Pillai, S.C.; Chu, W.; Hu, Y.; Jiang, X.; Fu, M.; Wu, X.; Li, F.; Wang, H. Visible light photocatalytic degradation of tetracycline with porous Ag/graphite carbon nitride plasmonic composite: Degradation pathways and mechanism. J. Colloid Interface Sci. 2020, 574, 110–121. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Seredych, M.; Rodríguez-Castellón, E.; Bandosz, T.J. Mesoporous Graphitic Carbon Nitride-Based Nanospheres as Visible-Light Active Chemical Warfare Agents Decontaminant. ChemNanoMat 2016, 2, 268–272. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Travlou, N.A.; Secor, J.; Bandosz, T.J. Oxidized g-C3N4 Nanospheres as Catalytically Photoactive Linkers in MOF/g-C3N4 Composite of Hierarchical Pore Structure. Small 2017, 13, 1601758. [Google Scholar] [CrossRef] [PubMed]
- Cerdan, K.; Ouyang, W.; Colmenares, J.C.; Muñoz-Batista, M.J.; Luque, R.; Balu, A.M. Facile mechanochemical modification of g-C3N4 for selective photo-oxidation of benzyl alcohol. Chem. Eng. Sci. 2019, 194, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Han, X.; Yu, H.; Zou, Y.; Dong, X. Enhanced photocatalytic performance of boron and phosphorous co-doped graphitic carbon nitride nanosheets for removal of organic pollutants. Sep. Purif. Technol. 2019, 226, 128–137. [Google Scholar] [CrossRef]
- You, R.; Dou, H.; Chen, L.; Zheng, S.; Zhang, Y. Graphitic carbon nitride with S and O codoping for enhanced visible light photocatalytic performance. RSC Adv. 2017, 7, 15842–15850. [Google Scholar] [CrossRef] [Green Version]
- Pawar, R.C.; Kang, S.; Park, J.H.; Kim, J.-H.; Ahn, S.; Lee, C.S. Room-temperature synthesis of nanoporous 1D microrods of graphitic carbon nitride (g-C3N4) with highly enhanced photocatalytic activity and stability. Sci. Rep. 2016, 6, 31147. [Google Scholar] [CrossRef]
- Florent, M.; Giannakoudakis, D.A.; Bandosz, T.J. Detoxification of mustard gas surrogate on ZnO2/g-C3N4 composites: Effect of surface features’ synergy and day-night photocatalysis. Appl. Catal. B Environ. 2020, 272, 119038. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Hu, Y.; Florent, M.; Bandosz, T.J. Smart textiles of MOF/g-C3N4 nanospheres for the rapid detection/detoxification of chemical warfare agents. Nanoscale Horiz. 2017, 2, 356–364. [Google Scholar] [CrossRef]
- Krivtsov, I.; García-López, E.I.; Marcì, G.; Palmisano, L.; Amghouz, Z.; García, J.R.; Ordóñez, S.; Díaz, E. Selective photocatalytic oxidation of 5-hydroxymethyl-2-furfural to 2,5-furandicarboxyaldehyde in aqueous suspension of g-C3N4. Appl. Catal. B Environ. 2017, 204, 430–439. [Google Scholar] [CrossRef]
- Wu, Q.; He, Y.; Zhang, H.; Feng, Z.; Wu, Y.; Wu, T. Photocatalytic selective oxidation of biomass-derived 5-hydroxymethylfurfural to 2,5-diformylfuran on metal-free g-C3N4 under visible light irradiation. Mol. Catal. 2017, 436, 10–18. [Google Scholar] [CrossRef]
- Ilkaeva, M.; Krivtsov, I.; García-López, E.I.; Marcì, G.; Khainakova, O.; García, J.R.; Palmisano, L.; Díaz, E.; Ordóñez, S. Selective photocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxaldehyde by polymeric carbon nitride-hydrogen peroxide adduct. J. Catal. 2018, 359, 212–222. [Google Scholar] [CrossRef] [Green Version]
- Battula, V.R.; Jaryal, A.; Kailasam, K. Visible light-driven simultaneous H2 production by water splitting coupled with selective oxidation of HMF to DFF catalyzed by porous carbon nitride. J. Mater. Chem. A 2019, 7, 5643–5649. [Google Scholar] [CrossRef]
- Bao, X.; Liu, M.; Wang, Z.; Dai, D.; Wang, P.; Cheng, H.; Liu, Y.; Zheng, Z.; Dai, Y.; Huang, B. Photocatalytic Selective Oxidation of HMF Coupled with H2 Evolution on Flexible Ultrathin g-C3N4 Nanosheets with Enhanced N–H Interaction. ACS Catal. 2022, 12, 1919–1929. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Y.; Chen, J.; Song, L.; Chen, L. One-Step Approach to 2,5-Diformylfuran from Fructose by Proton- and Vanadium-Containing Graphitic Carbon Nitride. ChemCatChem 2014, 6, 3174–3181. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.-J.; Liu, H.-Y.; Liu, J.-L.; Xu, G.-Y.; Liu, J.-X.; Sun, H.; Fu, Y. Graphitic Carbon Nitride (g-C3N4)-derived Fe-N-C Catalysts for Selective Hydrodeoxygenation of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran. ChemistrySelect 2017, 2, 11062–11070. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, Z.; Zhu, Y.; Wu, Y.; Wu, T. Photocatalytic selective oxidation of biomass-derived 5-hydroxymethylfurfural to 2,5-diformylfuran on WO3/g-C3N4 composite under irradiation of visible light. J. Photochem. Photobiol. A Chem. 2019, 371, 1–9. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Cheng, L.; Ismael, M.; Feng, Z.; Wu, Y. Novel application of g-C3N4/NaNbO3 composite for photocatalytic selective oxidation of biomass-derived HMF to FFCA under visible light irradiation. Adv. Powder Technol. 2020, 31, 1148–1159. [Google Scholar] [CrossRef]
- Cheng, L.; Huang, D.; Zhang, Y.; Wu, Y. Photocatalytic selective oxidation of HMF to DFF over Bi2WO6/mpg–C3N4 composite under visible light. Appl. Organomet. Chem. 2021, 35, e6404. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, S.; Arumugam, S.M.; Palanisami, M.; Shanmugam, V.; Elumalai, S. Nb2O5/g-C3N4 Heterojunction Facilitates 2,5-Diformylfuran Production via Photocatalytic Oxidation of 5-Hydroxymethylfurfural under Direct Sunlight Irradiation. ChemPhotoChem 2022, 6, e202100199. [Google Scholar] [CrossRef]
- Wang, X.-X.; Meng, S.; Zhang, S.; Zheng, X.; Chen, S. 2D/2D MXene/g-C3N4 for photocatalytic selective oxidation of 5-hydroxymethylfurfural into 2,5-formylfuran. Catal. Commun. 2020, 147, 106152. [Google Scholar] [CrossRef]
- Bandosz, T.J. Activated Carbon Surfaces in Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Janiszewska, D.; Olchowski, R.; Nowicka, A.; Zborowska, M.; Marszałkiewicz, K.; Shams, M.; Giannakoudakis, D.A.; Anastopoulos, I.; Barczak, M. Activated biochars derived from wood biomass liquefaction residues for effective removal of hazardous hexavalent chromium from aquatic environments. GCB Bioenergy 2021, 13, 1247–1259. [Google Scholar] [CrossRef]
- Wong, S.; Ngadi, N.; Inuwa, I.M.; Hassan, O. Recent advances in applications of activated carbon from biowaste for wastewater treatment: A short review. J. Clean. Prod. 2018, 175, 361–375. [Google Scholar] [CrossRef]
- Abatal, M.; Anastopoulos, I.; Giannakoudakis, D.A.; Olguin, M.T. Carbonaceous material obtained from bark biomass as adsorbent of phenolic compounds from aqueous solutions. J. Environ. Chem. Eng. 2020, 8, 103784. [Google Scholar] [CrossRef]
- Liakos, E.V.; Rekos, K.; Giannakoudakis, D.A.; Mitropoulos, A.C.; Fu, J.; Kyzas, G.Z. Activated Porous Carbon Derived from Tea and Plane Tree Leaves Biomass for the Removal of Pharmaceutical Compounds from Wastewaters. Antibiotics 2021, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Perciani de Moraes, N.; da Silva Rocha, R.; Caetano Pinto da Silva, M.L.; Bastos Campos, T.M.; Thim, G.P.; Landers, R.; Rodrigues, L.A. Facile preparation of Bi-doped ZnO/β-Bi2O3/Carbon xerogel composites towards visible-light photocatalytic applications: Effect of calcination temperature and bismuth content. Ceram. Int. 2020, 46, 23895–23909. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Wang, S.; Li, L. Dual templating fabrication of hierarchical porous three-dimensional ZnO/carbon nanocomposites for enhanced photocatalytic and photoelectrochemical activity. Appl. Catal. B Environ. 2018, 222, 209–218. [Google Scholar] [CrossRef]
- Im, J.-K.; Boateng, L.K.; Flora, J.R.V.; Her, N.; Zoh, K.-D.; Son, A.; Yoon, Y. Enhanced ultrasonic degradation of acetaminophen and naproxen in the presence of powdered activated carbon and biochar adsorbents. Sep. Purif. Technol. 2014, 123, 96–105. [Google Scholar] [CrossRef]
- Lisowski, P.; Colmenares, J.C.; Mašek, O.; Lisowski, W.; Lisovytskiy, D.; Kamińska, A.; Łomot, D. Dual Functionality of TiO2/Biochar Hybrid Materials: Photocatalytic Phenol Degradation in the Liquid Phase and Selective Oxidation of Methanol in the Gas Phase. ACS Sustain. Chem. Eng. 2017, 5, 6274–6287. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Vishnu, M.C.; Sharma, K.K.; Singh, R.; Madhav, S.; Tiwary, D.; Mishra, P.K. Comparative study of dye degradation using TiO2-activated carbon nanocomposites as catalysts in photocatalytic, sonocatalytic, and photosonocatalytic reactor. Desalin. Water Treat. 2016, 57, 20552–20564. [Google Scholar] [CrossRef]
- Davis, S.E.; Houk, L.R.; Tamargo, E.C.; Datye, A.K.; Davis, R.J. Oxidation of 5-hydroxymethylfurfural over supported Pt, Pd and Au catalysts. Catal. Today 2011, 160, 55–60. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Li, Z. CoOx-MC (MC = Mesoporous Carbon) for Highly Efficient Oxidation of 5-Hydroxymethylfurfural (5-HMF) to 2,5-Furandicarboxylic Acid (FDCA). ACS Sustain. Chem. Eng. 2020, 8, 4801–4808. [Google Scholar] [CrossRef]
- Gupta, D.; Pant, K.K.; Saha, B. Titania nanoparticles embedded in functionalized carbon for the aqueous phase oxidation of 5-hydroxymethylfurfural. Mol. Catal. 2017, 435, 182–188. [Google Scholar] [CrossRef]
- Lee, J.; Fortner, J.D.; Hughes, J.B.; Kim, J.-H. Photochemical Production of Reactive Oxygen Species by C60 in the Aqueous Phase During UV Irradiation. Environ. Sci. Technol. 2007, 41, 2529–2535. [Google Scholar] [CrossRef] [PubMed]
- Hasobe, T.; Hattori, S.; Kamat, P.V.; Fukuzumi, S. Supramolecular nanostructured assemblies of different types of porphyrins with fullerene using TiO2 nanoparticles for light energy conversion. Tetrahedron 2006, 62, 1937–1946. [Google Scholar] [CrossRef]
- Bai, X.; Wang, L.; Wang, Y.; Yao, W.; Zhu, Y. Enhanced oxidation ability of g-C3N4 photocatalyst via C60 modification. Appl. Catal. B Environ. 2014, 152–153, 262–270. [Google Scholar] [CrossRef]
- Li, G.; Jiang, B.; Li, X.; Lian, Z.; Xiao, S.; Zhu, J.; Zhang, D.; Li, H. C60/Bi2TiO4F2 Heterojunction Photocatalysts with Enhanced Visible-Light Activity for Environmental Remediation. ACS Appl. Mater. Interfaces 2013, 5, 7190–7197. [Google Scholar] [CrossRef]
- Han, G.; Jin, Y.-H.; Burgess, R.A.; Dickenson, N.E.; Cao, X.-M.; Sun, Y. Visible-Light-Driven Valorization of Biomass Intermediates Integrated with H2 Production Catalyzed by Ultrathin Ni/CdS Nanosheets. J. Am. Chem. Soc. 2017, 139, 15584–15587. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. Engl. 2016, 55, 8164–8215. [Google Scholar] [CrossRef] [Green Version]
- Azadi, P.; Inderwildi, O.R.; Farnood, R.; King, D.A. Liquid fuels, hydrogen and chemicals from lignin: A critical review. Renew. Sustain. Energy Rev. 2013, 21, 506–523. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Margellou, A.; Triantafyllidis, K.S. Catalytic Transfer Hydrogenolysis Reactions for Lignin Valorization to Fuels and Chemicals. Catalysts 2019, 9, 43. [Google Scholar] [CrossRef] [Green Version]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, P.A.; Fotopoulos, A.P.; Karakoulia, S.A.; Triantafyllidis, K.S. Catalytic Fast Pyrolysis of Kraft Lignin With Conventional, Mesoporous and Nanosized ZSM-5 Zeolite for the Production of Alkyl-Phenols and Aromatics. Front. Chem. 2018, 6, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charisteidis, I.; Lazaridis, P.; Fotopoulos, A.; Pachatouridou, E.; Matsakas, L.; Rova, U.; Christakopoulos, P.; Triantafyllidis, K. Catalytic Fast Pyrolysis of Lignin Isolated by Hybrid Organosolv—Steam Explosion Pretreatment of Hardwood and Softwood Biomass for the Production of Phenolics and Aromatics. Catalysts 2019, 9, 935. [Google Scholar] [CrossRef] [Green Version]
- Margellou, A.G.; Lazaridis, P.A.; Charisteidis, I.D.; Nitsos, C.K.; Pappa, C.P.; Fotopoulos, A.P.; Van den Bosch, S.; Sels, B.F.; Triantafyllidis, K.S. Catalytic fast pyrolysis of beech wood lignin isolated by different biomass (pre)treatment processes: Organosolv, hydrothermal and enzymatic hydrolysis. Appl. Catal. A Gen. 2021, 623, 118298. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Kang, T.C.; Wang, Z.Y.; Yoshikawa, T.; Nakasaka, Y.; Masuda, T.; Chuang, L.C.; Wu, K.C.W. Efficient liquid-phase hydrogenolysis of a lignin model compound (benzyl phenyl ether) using a Ni/carbon catalyst. React. Chem. Eng. 2019, 4, 618–626. [Google Scholar] [CrossRef]
- Cheng, C.B.; Li, P.F.; Yu, W.B.; Shen, D.K.; Jiang, X.X.; Gu, S. Nonprecious Metal/Bimetallic Catalytic Hydrogenolysis of Lignin in a Mixed-Solvent System. Acs Sustain. Chem. Eng. 2020, 8, 16217–16228. [Google Scholar] [CrossRef]
- Li, J.; Sun, H.; Liu, J.-X.; Zhang, J.-J.; Li, Z.-X.; Fu, Y. Selective reductive cleavage of C O bond in lignin model compounds over nitrogen-doped carbon-supported iron catalysts. Mol. Catal. 2018, 452, 36–45. [Google Scholar] [CrossRef]
- Oregui-Bengoechea, M.; Gandarias, I.; Arias, P.L.; Barth, T. Solvent and catalyst effect in the formic acid aided lignin-to-liquids. Bioresour. Technol. 2018, 270, 529–536. [Google Scholar] [CrossRef]
- Sanyoto, B.; Dwiatmoko, A.A.; Choi, J.W.; Ha, J.M.; Suh, D.J.; Kim, C.S.; Lim, J.C. Highly Dispersed Pt Nanoparticles for the Production of Aromatic Hydrocarbons by the Catalytic Degrading of Alkali Lignin. J. Nanosci. Nanotechnol. 2016, 16, 4565–4569. [Google Scholar] [CrossRef]
- Kristianto, I.; Limarta, S.O.; Lee, H.; Ha, J.M.; Suh, D.J.; Jae, J. Effective depolymerization of concentrated acid hydrolysis lignin using a carbon-supported ruthenium catalyst in ethanol/formic acid media. Bioresour. Technol. 2017, 234, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Shu, R.; Long, J.; Yuan, Z.; Zhang, Q.; Wang, T.; Wang, C.; Ma, L. Efficient and product-controlled depolymerization of lignin oriented by metal chloride cooperated with Pd/C. Bioresour. Technol. 2015, 179, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Shu, R.; Zhang, Q.; Ma, L.; Xu, Y.; Chen, P.; Wang, C.; Wang, T. Insight into the solvent, temperature and time effects on the hydrogenolysis of hydrolyzed lignin. Bioresour. Technol. 2016, 221, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Chen, Q.; Guo, H.W.; Hu, G.; Li, C.Z.; Wen, J.L.; Wang, H.S.; Zhang, T.; Zhao, Z.B.K.; Sun, R.C.; et al. Effects of Extraction Methods on Structure and Valorization of Corn Stover Lignin by a Pd/C Catalyst. ChemCatChem 2017, 9, 1135–1143. [Google Scholar] [CrossRef]
- Zhai, Y.; Li, C.; Xu, G.; Ma, Y.; Liu, X.; Zhang, Y.J.G.C. Depolymerization of lignin via a non-precious Ni–Fe alloy catalyst supported on activated carbon. Green Chem. 2017, 19, 1895–1903. [Google Scholar] [CrossRef]
- Wang, S.Z.; Gao, W.; Xiao, L.P.; Shi, J.; Sun, R.C.; Song, G.Y. Hydrogenolysis of biorefinery corncob lignin into aromatic phenols over activated carbon-supported nickel. Sustain. Energy Fuels 2019, 3, 401–408. [Google Scholar] [CrossRef]
- Cheng, C.; Li, P.; Yu, W.; Shen, D.; Gu, S. Catalytic hydrogenolysis of lignin in ethanol/isopropanol over an activated carbon supported nickel-copper catalyst. Bioresour. Technol. 2021, 319, 124238. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, J.F.; Cai, B.; Zhu, H.M.; Zhu, Y.Q.; Pan, H. Efficient Ni-Cu/AC Bimetal Catalyst for Hydrogenolysis of Lignin to Produce High-Value-Added Chemicals. ChemistrySelect 2020, 5, 10090–10097. [Google Scholar] [CrossRef]
- Xiao, L.P.; Wang, S.Z.; Li, H.L.; Li, Z.W.; Shi, Z.J.; Xiao, L.; Sun, R.G.; Fang, Y.M.; Song, G.Y. Catalytic Hydrogenolysis of Lignins into Phenolic Compounds over Carbon Nanotube Supported Molybdenum Oxide. ACS Catal. 2017, 7, 7535–7542. [Google Scholar] [CrossRef]
- Cao, Y.L.; Mao, S.J.; Li, M.M.; Chen, Y.Q.; Wang, Y. Metal/Porous Carbon Composites for Heterogeneous Catalysis: Old Catalysts with Improved Performance Promoted by N-Doping. ACS Catal. 2017, 7, 8090–8112. [Google Scholar] [CrossRef]
- Li, T.J.; Lin, H.F.; Ouyang, X.P.; Qiu, X.Q.; Wan, Z.C. In Situ Preparation of Ru@N-Doped Carbon Catalyst for the Hydrogenolysis of Lignin To Produce Aromatic Monomers. ACS Catal. 2019, 9, 5828–5836. [Google Scholar] [CrossRef]
- Li, T.; Lin, H.; Ouyang, X.; Qiu, X.; Wan, Z.; Ruan, T. Impact of nitrogen species and content on the catalytic activity to C–O bond cleavage of lignin over N-doped carbon supported Ru-based catalyst. Fuel 2020, 278, 118324. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Sharypov, V.I.; Baryshnikov, S.V.; Miroshnikova, A.V.; Taran, O.P.; Yakovlev, V.A.; Lavrenov, A.V.; Djakovitch, L. Catalytic hydrogenolysis of native and organosolv lignins of aspen wood to liquid products in supercritical ethanol medium. Catal. Today 2021, 379, 114–123. [Google Scholar] [CrossRef]
- Totong, S.; Daorattanachai, P.; Quitain, A.T.; Kida, T.; Laosiripojana, N. Catalytic Depolymerization of Alkaline Lignin into Phenolic-Based Compounds over Metal-Free Carbon-Based Catalysts. Ind. Eng. Chem. Res. 2019, 58, 13041–13052. [Google Scholar] [CrossRef]
- Wang, D.; Li, G.; Zhang, C.; Wang, Z.; Li, X. Nickel nanoparticles inlaid in lignin-derived carbon as high effective catalyst for lignin depolymerization. Bioresour. Technol. 2019, 289, 121629. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Ling, L.-L.; Jiang, H.J.G.C. Selective hydrogenation of lignin to produce chemical commodities by using a biochar supported Ni–Mo2C catalyst obtained from biomass. Green Chem. 2016, 18, 4032–4041. [Google Scholar] [CrossRef]
- Biswas, B.; Kumar, A.; Kaur, R.; Krishna, B.B.; Bhaskar, T. Catalytic hydrothermal liquefaction of alkali lignin over activated bio-char supported bimetallic catalyst. Bioresour. Technol. 2021, 337, 125439. [Google Scholar] [CrossRef]
- Lama, S.M.G.; Pampel, J.; Fellinger, T.P.; Beskoski, V.P.; Slavkovic-Beskoski, L.; Antonietti, M.; Molinari, V. Efficiency of Ni Nanoparticles Supported on Hierarchical Porous Nitrogen-Doped Carbon for Hydrogenolysis of Kraft Lignin in Flow and Batch Systems. Acs Sustain. Chem. Eng. 2017, 5, 2415–2420. [Google Scholar] [CrossRef]
- Herbst, A.; Janiak, C. MOF catalysts in biomass upgrading towards value-added fine chemicals. Crystengcomm 2017, 19, 4092–4117. [Google Scholar] [CrossRef] [Green Version]
- Si, X.-G.; Zhao, Y.-P.; Song, Q.-L.; Cao, J.-P.; Wang, R.-Y.; Wei, X.-Y.J.R.C. Hydrogenolysis of lignin-derived aryl ethers to monomers over a MOF-derived Ni/N–C catalyst. React. Chem. Eng. 2020, 5, 886–895. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Zhang, Z.; Li, M.; Yang, Q.; Yang, Y.; Bao, Z.; Ren, Q. M-Gallate (M = Ni, Co) Metal–Organic Framework-Derived Ni/C and Bimetallic Ni–Co/C Catalysts for Lignin Conversion into Monophenols. ACS Sustain. Chem. Eng. 2019, 7, 12955–12963. [Google Scholar] [CrossRef]
- Wang, Q.; Su, T.; Wang, Y.; Chen, Y.; Lu, X.; Ma, R.; Fu, Y.; Zhu, W. Metal–Organic Framework-Mediated Synthesis of One-Dimensional Nitrogen-Doped Molybdenum Carbide for the Cleavage of Lignin and Dimeric Lignin Model Compounds. ACS Sustain. Chem. Eng. 2020, 8, 17008–17015. [Google Scholar] [CrossRef]
- Ma, X.L.; Ma, R.; Hao, W.Y.; Chen, M.M.; Iran, F.; Cui, K.; Tian, Y.; Li, Y.D. Common Pathways in Ethanolysis of Kraft Lignin to Platform Chemicals over Molybdenum-Based Catalysts. ACS Catal. 2015, 5, 4803–4813. [Google Scholar] [CrossRef]
- Wu, K.; Yang, C.; Zhu, Y.; Wang, J.; Wang, X.; Liu, C.; Liu, Y.; Lu, H.; Liang, B.; Li, Y. Synthesis-Controlled α- and β-Molybdenum Carbide for Base-Promoted Transfer Hydrogenation of Lignin to Aromatic Monomers in Ethanol. Ind. Eng. Chem. Res. 2019, 58, 20270–20281. [Google Scholar] [CrossRef]
- Yang, X.J.; Feng, M.Q.; Choi, J.S.; Meyer, H.M.; Yang, B. Depolymerization of corn stover lignin with bulk molybdenum carbide catalysts. Fuel 2019, 244, 528–535. [Google Scholar] [CrossRef]
- Yan, B.; Lin, X.; Chen, Z.; Cai, Q.; Zhang, S. Selective production of phenolic monomers via high efficient lignin depolymerization with a carbon based nickel-iron-molybdenum carbide catalyst under mild conditions. Bioresour. Technol. 2021, 321, 124503. [Google Scholar] [CrossRef]
- Guo, H.; Qi, Z.; Liu, Y.; Xia, H.; Li, L.; Huang, Q.; Wang, A.; Li, C. Tungsten-based catalysts for lignin depolymerization: The role of tungsten species in C–O bond cleavage. Catal. Sci. Technol. 2019, 9, 2144–2151. [Google Scholar] [CrossRef]
- Gomez-Monedero, B.; Ruiz, M.P.; Bimbela, F.; Faria, J. Selective depolymerization of industrial lignin-containing stillage obtained from cellulosic bioethanol processing. FuelProcess. Technol. 2018, 173, 165–172. [Google Scholar] [CrossRef]
- Jia, P.F.; Wang, J.; Zhang, W.L. Catalytic hydrothermal liquefaction of lignin over carbon nanotube supported metal catalysts for production of monomeric phenols. J. Energy Inst. 2021, 94, 1–10. [Google Scholar] [CrossRef]










| Catalyst | Sono (S) or SonoPhoto (SP) | COMPOUND | Experimental Conditions | Degradation (%) | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Catalytic Loading (g/L) | Frequency of Sonication (kHz) | Power of Sonication (W) | Duration (Min) | Light Source | Concentration | Temperature (°C) | |||||
| single walled carbon nanotubes (SWNTs) | S | ibuprofen | 0.045 | 1000 | 180 | 60 | - | 50 mg/L | 15 | 97 | [64] |
| sulfamethoxazole | 0.045 | 1000 | 180 | 60 | - | 45 mg/L | 15 | 92 | |||
| mMBiPO4-MWCNTs-In2O3 | S | Norfloxacin | 1 | 40 | 300 | 150 | - | 10 mg/L | 25–28 | 69% | [65] |
| F-TiO2 (B)/SWCNT | SP | malachite green | 0.1 | 45−55 Hz | 285 | 120 | 500 W halogen lamp | 30 mg/L | 20 | 95 | [66] |
| P | 0.1 | - | - | 120 | 500 W halogen lamp | 30 mg/L | 20 | 91 | |||
| TiO2/CNTs | SP | methyl orange | 1 | 20 | 50 | 60 | 30 W black light blue lamp | 25 ppm | - | 66 | [67] |
| rGO/Ag2CO3 | SP | Tetracycline | 0.3 | 20 | - | 60 | 500 W xenon light source | 10 ppm | 20 | 97 | [68] |
| Au/BeTiO2/rGO | SP | Tetracycline | 0.25 | 40 | 600 | 60 | 300 W halogen lamp | 15 | - | 100 | [69] |
| NiFe-LDH/rGO | SP | moxifloxacin | 1 | 36 | 150 | 60 | 10 W LED lamp | 20 mg/L | Room tem | 90 | [70] |
| ZnCr LDH/rGO | SP | Rifampicin | 1.5 | 36 | 150 | 60 | 10 W LED vis | 15 mg/L | Room tem | 87 | [71] |
| ZnCr LDH/BC | SP | Rifampicin | 0.6 | 36 | 150 | 40 | 30 W LED vis | 15 mg/L | - | 98 | [72] |
| TiO2/coconut shell-derived Activated carbon | S | Methylene blue | 2.5 | 26 | 40 | 120 | - | 10 mg/L | 25 | 50 | [73] |
| TiO2 decorated on magnetic activated carbon (MAC@T) | SP | tetra-cycline | 0.4 | 20 | 70 | 180 | UV (254 nm) | 25 | 20 | 93 | [74] |
| TiO2/BC | S | Methylene blue | 1.5 | 40 | 300 | 80 | - | 20 | room | 98 | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannakoudakis, D.A.; Zormpa, F.F.; Margellou, A.G.; Qayyum, A.; Colmenares-Quintero, R.F.; Len, C.; Colmenares, J.C.; Triantafyllidis, K.S. Carbon-Based Nanocatalysts (CnCs) for Biomass Valorization and Hazardous Organics Remediation. Nanomaterials 2022, 12, 1679. https://doi.org/10.3390/nano12101679
Giannakoudakis DA, Zormpa FF, Margellou AG, Qayyum A, Colmenares-Quintero RF, Len C, Colmenares JC, Triantafyllidis KS. Carbon-Based Nanocatalysts (CnCs) for Biomass Valorization and Hazardous Organics Remediation. Nanomaterials. 2022; 12(10):1679. https://doi.org/10.3390/nano12101679
Chicago/Turabian StyleGiannakoudakis, Dimitrios A., Foteini F. Zormpa, Antigoni G. Margellou, Abdul Qayyum, Ramón Fernando Colmenares-Quintero, Christophe Len, Juan Carlos Colmenares, and Konstantinos S. Triantafyllidis. 2022. "Carbon-Based Nanocatalysts (CnCs) for Biomass Valorization and Hazardous Organics Remediation" Nanomaterials 12, no. 10: 1679. https://doi.org/10.3390/nano12101679