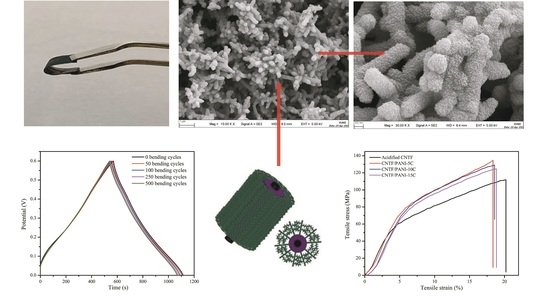
Continuously Reinforced Carbon Nanotube Film Sea-Cucumber-like Polyaniline Nanocomposites for Flexible Self-Supporting Energy-Storage Electrode Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CNTF
2.3. Preparation of Flexible Self-Supporting CNTF/PANI Nanocomposite Films
2.4. Characterization
2.5. Electrochemical Measurements
3. Results and Discussion
3.1. Structural, Morphological, and Textual Analyses
3.2. Electrochemical Performance Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, R.; Chang, Y.; Yang, X.; Dai, J.; Chen, Y.; Chang, W.; Xiong, W. Electromechanical sorting method for improving the sensitivity of micropyramid carbon nanotube film flexible force sensor. Compos. Part B Eng. 2021, 217, 108818. [Google Scholar] [CrossRef]
- Dubal, D.P.; Chodankar, N.R.; Kim, D.H.; Gomez-Romero, P. Towards flexible solid-state supercapacitors for smart and wearable electronics. Chem. Soc. Rev. 2018, 47, 2065–2129. [Google Scholar] [CrossRef]
- Gao, J.; Wang, X.; Zhai, W.; Liu, H.; Zheng, G.; Dai, K.; Mi, L.; Liu, C.; Shen, C. Ultrastretchable Multilayered Fiber with a Hollow-Monolith Structure for High-Performance Strain Sensor. ACS Appl. Mater. Interfaces 2018, 10, 34592–34603. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.P.; Mukhiya, T.; Muthurasu, A.; Chhetri, K.; Lee, M.; Dahal, B.; Lohani, P.C.; Kim, H.Y. A Review of Electrospun Carbon Nanofiber-Based Negative Electrode Materials for Supercapacitors. Electrochem 2021, 2, 236–250. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Hu, J.; Jung, E.; Zhang, J.; Jun, S.C.; Yamauchi, Y. Unlocking the Potential of Oxygen-Deficient Copper-Doped Co3O4 Nanocrystals Confined in Carbon as an Advanced Electrode for Flexible Solid-State Supercapacitors. ACS Energy Lett. 2021, 6, 3011–3019. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Zhang, J.; Jung, E.; Lee, S.; Jun, S.C. Structural engineering and surface modification of MOF-derived cobalt-based hybrid nanosheets for flexible solid-state supercapacitors. Energy Storage Mater. 2020, 32, 167–177. [Google Scholar] [CrossRef]
- Breczko, J.; Grzeskiewicz, B.; Gradzka, E.; Bobrowska, D.M.; Basa, A.; Goclon, J.; Winkler, K. Synthesis of polyaniline nanotubes decorated with graphene quantum dots: Structural & electrochemical studies. Electrochim. Acta 2021, 388, 138614. [Google Scholar]
- Yin, S.; Liu, X.; Kobayashi, Y.; Nishina, Y.; Nakagawa, R.; Yanai, R.; Kimura, K.; Miyake, T. A needle-type biofuel cell using enzyme/mediator/carbon nanotube composite fibers for wearable electronics. Biosens. Bioelectron. 2020, 165, 112287. [Google Scholar] [CrossRef]
- Chen, M.; Hu, X.; Li, K.; Sun, J.; Liu, Z.; An, B.; Zhou, X.; Liu, Z. Self-assembly of dendritic-lamellar MXene/Carbon nanotube conductive films for wearable tactile sensors and artificial skin. Carbon 2020, 164, 111–120. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Jun, S.C. Challenges and Strategies toward Cathode Materials for Rechargeable Potassium-Ion Batteries. Adv. Mater. 2021, 33, e2004689. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Zhou, Y.; Ou, K.; Dai, Y.; You, X.; Wang, H.; He, J.; Qin, X.; Wang, R. Weavable and stretchable piezoresistive carbon nanotubes-embedded nanofiber sensing yarns for highly sensitive and multimodal wearable textile sensor. Carbon 2020, 170, 464–476. [Google Scholar] [CrossRef]
- Dong, K.; Peng, X.; Wang, Z.L. Fiber/Fabric-Based Piezoelectric and Triboelectric Nanogenerators for Flexible/Stretchable and Wearable Electronics and Artificial Intelligence. Adv. Mater. 2020, 32, 1902549. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, G.; Yan, B.; Suo, H.; Zhao, C. Significant enhancement of electrochemical behaviour by incorporation of carboxyl group functionalized carbon nanotubes into polyaniline based supercapacitor. Eur. Polym. J. 2016, 83, 53–59. [Google Scholar] [CrossRef]
- Hussain, S.; Kovacevic, E.; Amade, R.; Berndt, J.; Pattyn, C.; Dias, A.; Boulmer-Leborgne, C.; Ammar, M.-R.; Bertran-Serra, E. Plasma synthesis of polyaniline enrobed carbon nanotubes for electrochemical applications. Electrochim. Acta 2018, 268, 218–225. [Google Scholar] [CrossRef]
- Salvatierra, R.V.; Oliveira, M.M.; Zarbin, A.J.G. One-Pot Synthesis and Processing of Transparent, Conducting, and Freestanding Carbon Nanotubes/Polyaniline Composite Films. Chem. Mater. 2010, 22, 5222–5234. [Google Scholar] [CrossRef]
- Yanilmaz, M.; Dirican, M.; Asiri, A.M.; Zhang, X. Flexible polyaniline-carbon nanofiber supercapacitor electrodes. J. Energy Storage 2019, 24, 100766. [Google Scholar] [CrossRef]
- Hsieh, Y.Y.; Zhang, Y.; Zhang, L.; Fang, Y.; Kanakaraaj, S.N.; Bahk, J.H.; Shanov, V. High thermoelectric power-factor composites based on flexible three-dimensional graphene and polyaniline. Nanoscale 2019, 11, 6552–6560. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, F.; Jia, C.; Mu, K.; Yu, M.; Lv, Y.; Shao, Z. Nitrogen and oxygen-codoped carbon nanospheres for excellent specific capacitance and cyclic stability supercapacitor electrodes. Chem. Eng. J. 2017, 330, 1166–1173. [Google Scholar] [CrossRef]
- Liu, D.; Wang, H.; Du, P.; Liu, P. Independently double-crosslinked carbon nanotubes/polyaniline composite films as flexible and robust free-standing electrodes for high-performance supercapacitors. Carbon 2017, 122, 761–774. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, L.; Xiao, X.; Yao, B.; Yuan, L.; Li, T.; Hu, Z.; Wang, B.; Wan, J.; Zhou, J. Flexible and cross-linked N-doped carbon nanofiber network for high performance freestanding supercapacitor electrode. Nano Energy 2015, 15, 66–74. [Google Scholar] [CrossRef]
- Liu, D.; Liu, J.; Wang, Q.; Du, P.; Wei, W.; Liu, P. PANI coated microporous graphene fiber capable of subjecting to external mechanical deformation for high performance flexible supercapacitors. Carbon 2019, 143, 147–153. [Google Scholar] [CrossRef]
- Ge, D.; Yang, L.; Fan, L.; Zhang, C.; Xiao, X.; Gogotsi, Y.; Yang, S. Foldable supercapacitors from triple networks of macroporous cellulose fibers, single-walled carbon nanotubes and polyaniline nanoribbons. Nano Energy 2015, 11, 568–578. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, Y.; Yao, Z.; Liu, A.; Shi, G. Supercapacitors Based on Flexible Graphene/Polyaniline Nanofiber Composite Films. ACS Nano 2010, 4, 1963–1970. [Google Scholar] [CrossRef]
- Wu, D.; Zhong, W. A new strategy for anchoring a functionalized graphene hydrogel in a carbon cloth network to support a lignosulfonate/polyaniline hydrogel as an integrated electrode for flexible high areal-capacitance supercapacitors. J. Mater. Chem. A 2019, 7, 5819–5830. [Google Scholar] [CrossRef]
- Yong, Y.; Dong, X.; Chan-Park, M.B.; Song, H.; Chen, P. Macroporous and Monolithic Anode Based on Polyaniline Hybridized Three-Dimensional Graphene for High-Performance Microbial Fuel Cells. ACS Nano 2012, 6, 2394–2400. [Google Scholar] [CrossRef]
- Lyu, S.; Chen, Y.; Zhang, L.; Han, S.; Lu, Y.; Chen, Y.; Yang, N.; Chen, Z.; Wang, S. Nanocellulose supported hierarchical structured polyaniline/nanocarbon nanocomposite electrode via layer-by-layer assembly for green flexible supercapacitors. RSC Adv. 2019, 9, 17824–17834. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; He, K.; Wu, Y.; Mao, J.; Yang, Z.; Xu, Y.; Yuan, C.; Zeng, B.; Dai, L. A high performance flexible recyclable supercapacitor with polyaniline by casting in unconventional proportion. J. Power Sources 2019, 442, 227215. [Google Scholar] [CrossRef]
- Han, S.; Wu, D.; Li, S.; Zhang, F.; Feng, X. Porous graphene materials for advanced electrochemical energy storage and conversion devices. Adv. Mater. 2014, 26, 849–864. [Google Scholar] [CrossRef]
- Wickramaarachchi, W.A.M.K.P.; Minakshi, M.; Gao, X.; Dabare, R.; Wong, K.W. Hierarchical porous carbon from mango seed husk for electro-chemical energy storage. Chem. Eng. J. Adv. 2021, 8, 100158. [Google Scholar] [CrossRef]
- Liu, R.; Sun, S.; Zhong, R.; Zhang, H.; Wu, X. Nitrogen-doped microporous carbon coated on carbon nanotubes for high performance supercapacitors. Micropor. Mesopor. Mater. 2020, 305, 110300. [Google Scholar] [CrossRef]
- Zhai, Y.; Dou, Y.; Zhao, D.; Fulvio, P.F.; Mayes, R.T.; Dai, S. Carbon materials for chemical capacitive energy storage. Adv. Mater. 2011, 23, 4828–4850. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.; Elanthamilan, E.; Princy Merlin, J.; Sathiyan, A. Enhanced electrochemical behaviour of FeCo2O4/PANI electrode material for supercapacitors. J. Alloy. Compd. 2021, 874, 159876. [Google Scholar] [CrossRef]
- Zhang, W.; Kong, Y.; Jin, X.; Yan, B.; Diao, G.; Piao, Y. Supramolecule-assisted synthesis of cyclodextrin polymer functionalized polyaniline/carbon nanotube with core-shell nanostructure as high-performance supercapacitor material. Electrochim. Acta 2020, 331, 135345. [Google Scholar] [CrossRef]
- Li, X.; Tang, Y.; Song, J.; Yang, W.; Wang, M.; Zhu, C.; Zhao, W.; Zheng, J.; Lin, Y. Self-supporting activated carbon/carbon nanotube/reduced graphene oxide flexible electrode for high performance supercapacitor. Carbon 2018, 129, 236–244. [Google Scholar] [CrossRef]
- Xu, L.; Peng, Q.; Zhu, Y.; Zhao, X.; Yang, M.; Wang, S.; Xue, F.; Yuan, Y.; Lin, Z.; Xu, F.; et al. Artificial muscle with reversible and controllable deformation based on stiffness-variable carbon nanotube spring-like nanocomposite yarn. Nanoscale 2019, 11, 8124–8132. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, Y.; Suresh, L.; Hao, A.; Bick, M.; Tan, S.C.; Chen, J. Carbon Nanotube Reinforced Strong Carbon Matrix Composites. ACS Nano 2020, 14, 9282–9319. [Google Scholar] [CrossRef]
- Che, B.; Li, H.; Zhou, D.; Zhang, Y.; Zeng, Z.; Zhao, C.; He, C.; Liu, E.; Lu, X. Porous polyaniline/carbon nanotube composite electrode for supercapacitors with outstanding rate capability and cyclic stability. Compos. Part B Eng. 2019, 165, 671–678. [Google Scholar] [CrossRef]
- Guan, L.; Yu, L.; Chen, G.Z. Capacitive and non-capacitive faradaic charge storage. Electrochim. Acta 2016, 206, 464–478. [Google Scholar] [CrossRef]
- Jabeen, N.; Xia, Q.; Yang, M.; Xia, H. Unique Core-Shell Nanorod Arrays with Polyaniline Deposited into Mesoporous NiCo2O4 Support for High-Performance Supercapacitor Electrodes. ACS Appl. Mater. Interfaces 2016, 8, 6093–6100. [Google Scholar] [CrossRef]
- Rauhala, T.; Davodi, F.; Sainio, J.; Sorsa, O.; Kallio, T. On the stability of polyaniline/carbon nanotube composites as binder-free positive electrodes for electrochemical energy storage. Electrochim. Acta 2020, 336, 135735. [Google Scholar] [CrossRef]
- Aydinli, A.; Yuksel, R.; Unalan, H.E. Vertically aligned carbon nanotube-Polyaniline nanocomposite supercapacitor electrodes. Int. J. Hydrog. Energy 2018, 43, 18617–18625. [Google Scholar] [CrossRef]
- Guo, Y.; Li, L.; Zhao, C.; Song, L.; Wang, B. Humidity sensing properties of poly-vanadium-titanium acid combined with polyaniline grown in situ by electrochemical polymerization. Sens. Actuators B Chem. 2018, 270, 80–88. [Google Scholar] [CrossRef]
- Liu, D.; Du, P.; Wei, W.; Wang, H.; Wang, Q.; Liu, P. Flexible and Robust Sandwich-Structured S-Doped Reduced Graphene Oxide/Carbon Nanotubes/Polyaniline (S-rGO/CNTs/PANI) Composite Membranes: Excellent Candidate as Free-Standing Electrodes for High-Performance Supercapacitors. Electrochim. Acta 2017, 233, 201–209. [Google Scholar] [CrossRef]
- Cao, M.S.; Yang, J.; Song, W.L.; Zhang, D.Q.; Wen, B.; Jin, H.B.; Hou, Z.L.; Yuan, J. Ferroferric oxide/multiwalled carbon nanotube vs polyaniline/ferroferric oxide/multiwalled carbon nanotube multiheterostructures for highly effective microwave absorption. ACS Appl. Mater. Interfaces 2012, 4, 6949–6956. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Z.; Jing, S.; Zhuo, H.; Hu, Y.; Liu, J.; Zhong, L.; Peng, X.; Liu, C. A foldable composite electrode with excellent electrochemical performance using microfibrillated cellulose fibers as a framework. J. Mater. Chem. A 2018, 6, 20338–20346. [Google Scholar] [CrossRef]
- Wang, H.; Liu, D.; Du, P.; Liu, P. Flexible and robust amino-functionalized glass fiber filter paper/polyaniline composite films as free-standing tensile-tolerant electrodes for high performance supercapacitors. Electrochim. Acta 2017, 228, 371–379. [Google Scholar] [CrossRef]
- Bhandari, S.; Singha, N.K.; Khastgir, D. Synthesis of graphene-like ultrathin polyaniline and its post-polymerization coating on nanosilica leading towards superhydrophobicity of composites. Chem. Eng. J. 2017, 313, 1302–1310. [Google Scholar] [CrossRef]
- Li, M.; Gao, Y.; Chen, N.; Meng, X.; Wang, C.; Zhang, Y.; Zhang, D.; Wei, Y.; Du, F.; Chen, G. Cu3V2O8 nanoparticles as intercalation-type anode material for lithium-ion batteries. Chem. Eur. J. 2016, 22, 11405–11412. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, B.; Zhou, Y.; Xu, X.; Yang, R.; Wang, Q.; Li, J. Hierarchical N-doped holey three-dimensional reduced graphene oxide with high performance capacitive deionization. J. Mater. Res. Technol. 2021, 15, 1996–2006. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Zhang, J.; Jun, S.C.; Yamauchi, Y. Carbonaceous Anode Materials for Non-aqueous Sodium- and Potassium-Ion Hybrid Capacitors. ACS Energy Lett. 2021, 6, 4127–4154. [Google Scholar] [CrossRef]
- Das, A.; Pisana, S.; Chakraborty, B.; Piscanec, S.; Saha, S.K.; Waghmare, U.V.; Novoselov, K.S.; Krishnamurthy, H.R.; Geim, A.K.; Ferrari, A.C.; et al. Monitoring dopants by Raman scattering in an electrochemically top-gated graphene transistor. Nat. Nanotechnol. 2008, 3, 210–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Spatially Resolved Raman Spectroscopy of Single- and Few-Layer Graphene. Nano Lett. 2007, 7, 238–242. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.J.; Sharma, I.; Elizabeth, I.; Gahtori, B.; Titus, S.S.; Chandra, P.; Gupta, B.K. A Comparative Study of Compressible and Conductive Vertically Aligned Carbon Nanotube Forest in Different Polymer Matrixes for High-Performance Piezoresistive Force Sensors. ACS Appl. Mater. Interfaces 2020, 12, 16946–16958. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Shao, C.; Li, X.; Lu, N.; Wang, K.; Zhang, X.; Liu, Y. Polyaniline-coated electrospun carbon nanofibers with high mass loading and enhanced capacitive performance as freestanding electrodes for flexible solid-state supercapacitors. Energy 2016, 95, 233–241. [Google Scholar] [CrossRef]
- Jeyaranjan, A.; Sakthivel, T.S.; Neal, C.J.; Seal, S. Scalable ternary hierarchical microspheres composed of PANI/rGO/CeO2 for high performance supercapacitor applications. Carbon 2019, 151, 192–202. [Google Scholar] [CrossRef]
- Zhou, S.-X.; Tao, X.-Y.; Ma, J.; Qu, C.-H.; Zhou, Y.; Guo, L.-T.; Feng, P.-Z.; Zhu, Y.-B.; Wei, X.-Y. Facile synthesis of self-assembled polyaniline nanorods doped with sulphuric acid for high-performance supercapacitors. Vacuum 2017, 143, 63–70. [Google Scholar] [CrossRef]
- Liu, S.; Liu, L.; Guo, H.; Oguzie, E.E.; Li, Y.; Wang, F. Electrochemical polymerization of polyaniline-reduced graphene oxide composite coating on 5083 Al alloy: Role of reduced graphene oxide. Electrochem. Commun. 2019, 98, 110–114. [Google Scholar] [CrossRef]
- Guo, F.; Xiao, P.; Yan, B.; Hahn, M.; Kong, Y.; Zhang, W.; Piao, Y.; Diao, G. One-pot synthesis of hydrazide-pillar[5]arene functionalized reduced graphene oxide for supercapacitor electrode. Chem. Eng. J. 2020, 391, 123511. [Google Scholar] [CrossRef]
- Lu, Q.; Zhao, Q.; Zhang, H.; Li, J.; Wang, X.; Wang, F. Water Dispersed Conducting Polyaniline Nanofibers for High-Capacity Rechargeable Lithium–Oxygen Battery. ACS Macro Lett. 2013, 2, 92–95. [Google Scholar] [CrossRef]
- Liu, D.; Wang, H.; Du, P.; Wei, W.; Wang, Q.; Liu, P. Flexible and robust reduced graphene oxide/carbon nanoparticles/polyaniline (RGO/CNs/PANI) composite films: Excellent candidates as free-standing electrodes for high-performance supercapacitors. Electrochim. Acta 2018, 259, 161–169. [Google Scholar] [CrossRef]
- Mondal, S.; Rana, U.; Malik, S. Reduced Graphene Oxide/Fe3O4/Polyaniline Nanostructures as Electrode Materials for an All-Solid-State Hybrid Supercapacitor. J. Phys. Chem. C 2017, 121, 7573–7583. [Google Scholar] [CrossRef]
- Cao, M.; Feng, Y.; Tian, R.; Chen, Q.; Chen, J.; Jia, M.; Yao, J. Free-standing porous carbon foam as the ultralight and flexible supercapacitor electrode. Carbon 2020, 161, 224–230. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.; Du, P.; Liu, J.; Liu, D.; Liu, P. Porous polylactic acid/carbon nanotubes/polyaniline composite film as flexible free-standing electrode for supercapacitors. Electrochim. Acta 2019, 294, 312–324. [Google Scholar] [CrossRef]
- Wang, H.; Liu, D.; Du, P.; Liu, P. Facile deposition of polyaniline on the multi-walled carbon nanotubes/polyvinyl chloride composite films as flexible and robust electrodes for high performance supercapacitors. Electrochim. Acta 2018, 289, 104–111. [Google Scholar] [CrossRef]
- Ahirrao, D.J.; Pal, A.K.; Singh, V.; Jha, N. Nanostructured porous polyaniline (PANI) coated carbon cloth (CC) as electrodes for flexible supercapacitor device. J. Mater. Sci. Technol. 2021, 88, 168–182. [Google Scholar] [CrossRef]
- Sundaram, M.M.; Watcharatharapong, T.; Chakraborty, S.; Ahuja, R.; Duraisamy, S.; Rao, P.T.; Munichandraiah, N. Synthesis, and crystal and electronic structure of sodium metal phosphate for use as a hybrid capacitor in non-aqueous electrolyte. Dalton Trans. 2015, 44, 20108–20120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minakshi, M.; Mitchell, D.R.G.; Munnangi, A.R.; Barlow, A.J.; Fichtner, M. New insights into the electrochemistry of magnesium molybdate hierarchical architectures for high performance sodium devices. Nanoscale 2018, 10, 13277–13288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, M.L.; Minakshi, M.; Singh, N.K. Synthesis and Characterization of Solid Polymer Electrolyte based on Activated Carbon for Solid State Capacitor. Electrochim. Acta 2014, 137, 497–503. [Google Scholar] [CrossRef] [Green Version]










| Samples | Cycles (c) | Scan Rate (mV/s) | PANI Content (wt%) | Thickness (μm) | C (1 A/g) (F/g) | E Wh/kg |
|---|---|---|---|---|---|---|
| CNTF | - | - | 0 | 15.0 | 25.6 | 1.2 |
| Acidified CNTF | - | - | 0 | 15.0 | 88.9 | 4.4 |
| CNTF/PANI-1C | 1 | 10 | 62.5 | 22.6 | 172.3 | 8.6 |
| CNTF/PANI-3C | 3 | 10 | 73.2 | 26.3 | 464.8 | 23.2 |
| CNTF/PANI-5C | 5 | 10 | 121.5 | 30.7 | 565.0 | 28.2 |
| CNTF/PANI-10C | 10 | 10 | 163.1 | 51.1 | 700.1 | 35.0 |
| CNTF/PANI-15C | 15 | 10 | 441.1 | 143.1 | 903.6 | 45.2 |
| Samples | Current Density (A/g) | Electrolyte | Specific Capacitance (F/g) | Cycle Stability | Tensile Strength (MPa) | Ref. |
|---|---|---|---|---|---|---|
| C-CNTs/TC-PANI | 1.0 | 1 M H2SO4 | 531.0 | 92.3% (1000C) | 1.75 | [19] |
| RGO/CNs/PANI | 1.0 | 1 M H2SO4 | 787.3 | 92.2% (2000C) | 10.1 | [60] |
| rGO/Fe3O4/PANI | 1.0 | 0.5 M H3PO4 | 283.4 | 78.0% (5000C) | 9.2 | [61] |
| CMS@CZL | 1.0 | 6 M KOH | 238.0 | 94.3% (10,000C) | 0.08 | [62] |
| PLA/CNTs/PANI | 1.0 | 1 M H2SO4 | 510.3 | 115.0% (2000C) | 18.7 | [63] |
| PANI/MWCNTs/PVC | 1.0 | 1 M H2SO4 | 801.1 | 90.6% (2000C) | 44.1 | [64] |
| PANI-CC | 1.0 | 1 M H2SO4 | 691.0 | 94.0% (2000C) | - | [65] |
| CNTF/PANI-15C | 1.0 | 1 M H2SO4 | 903.6 | 95.1% (2000C) | 124.5 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Liu, S.; Yang, H.; Xu, X.; Zhou, Y.; Yang, R.; Zhang, Y.; Li, J. Continuously Reinforced Carbon Nanotube Film Sea-Cucumber-like Polyaniline Nanocomposites for Flexible Self-Supporting Energy-Storage Electrode Materials. Nanomaterials 2022, 12, 8. https://doi.org/10.3390/nano12010008
Li B, Liu S, Yang H, Xu X, Zhou Y, Yang R, Zhang Y, Li J. Continuously Reinforced Carbon Nanotube Film Sea-Cucumber-like Polyaniline Nanocomposites for Flexible Self-Supporting Energy-Storage Electrode Materials. Nanomaterials. 2022; 12(1):8. https://doi.org/10.3390/nano12010008
Chicago/Turabian StyleLi, Bingjian, Shi Liu, Haicun Yang, Xixi Xu, Yinjie Zhou, Rong Yang, Yun Zhang, and Jinchun Li. 2022. "Continuously Reinforced Carbon Nanotube Film Sea-Cucumber-like Polyaniline Nanocomposites for Flexible Self-Supporting Energy-Storage Electrode Materials" Nanomaterials 12, no. 1: 8. https://doi.org/10.3390/nano12010008