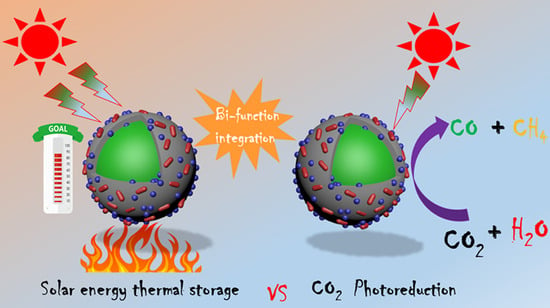
Bi-Functional Paraffin@Polyaniline/TiO2/PCN-222(Fe) Microcapsules for Solar Thermal Energy Storage and CO2 Photoreduction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphology Structure of PPTP MEPCMs
2.2. Chemical Composition Characterization
2.3. Pore Structure and Specific Surface Area Analysis
2.4. Phase Change and Thermal Properties Analysis
2.5. Photothermal Conversion Performance
2.6. Photoelectric Performance
2.7. Photocatalytic Performance of Catalysts for CO2 Photoreduction
2.8. Photocatalysis Mechanism of PPTP-3 MEPCM for CO2 Photoreduction
2.9. Performance Comparison of MEPCMs with Organic−Inorganic Hybrid Shells in the Literature
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chilvers, J.; Bellamy, R.; Pallett, H.; Hargreaves, T. A systemic approach to mapping participation with low-carbon energy transitions. Nat. Energy 2021, 6, 250–259. [Google Scholar] [CrossRef]
- Weck, P.F.; Kim, E. Solar Energy storage in phase change materials: First-principles thermodynamic modeling of magnesium chloride hydrates. J. Phys. Chem. C 2014, 118, 4618–4625. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, D. Innovative design of microencapsulated phase change materials for thermal energy storage and versatile applications: A review. Sustain. Energy Fuels 2019, 3, 1091–1149. [Google Scholar] [CrossRef]
- Bazri, S.; Badruddin, I.A.; Naghavi, M.S.; Bahiraei, M. A review of numerical studies on solar collectors integrated with latent heat storage systems employing fins or nanoparticles. Renew. Energy 2018, 118, 761–778. [Google Scholar] [CrossRef]
- Akhiani, A.R.; Mehrali, M.; Latibari, S.T.; Mehrali, M.; Mahlia, T.M.I.; Sadeghinezhad, E.; Metselaar, H.S.C. One-step preparation of form-stable phase change material through self-assembly of fatty acid and graphene. J. Phys. Chem. C 2015, 119, 22787–22796. [Google Scholar] [CrossRef]
- Lu, Y.; Xiao, X.; Liu, Y.; Wang, J.; Qi, S.; Huan, C.; Liu, H.; Zhu, Y.; Xu, G. Achieving multifunctional smart textile with long afterglow and thermo-regulation via coaxial electrospinning. J. Alloy. Compd. 2020, 812, 152144. [Google Scholar] [CrossRef]
- Mehrali, M.; Johan, E.; Shahi, M.; Mahmoudi, A. Simultaneous solar-thermal energy harvesting and storage via shape stabilized salt hydrate phase change material. Chem. Eng. J. 2021, 405, 126624. [Google Scholar] [CrossRef]
- Li, X.; Sun, L.; Sui, H.; He, L.; Yuan, W.; Han, Z. A novel polymeric adsorbent embedded with phase change materials (PCMs) microcapsules: Synthesis and application. Nanomaterials 2019, 9, 736. [Google Scholar] [CrossRef] [Green Version]
- Woo, H.Y.; Lee, D.W.; Yoon, T.Y.; Kim, J.B.; Chae, J.-Y.; Paik, T. Sub-100-nm nearly monodisperse n-paraffin/PMMA phase change nanobeads. Nanomaterials 2021, 11, 204. [Google Scholar] [CrossRef]
- Kong, L.; Kong, X.; Ji, Z.; Wang, X.; Zhang, X. Large-scale fabrication of a robust superhydrophobic thermal energy storage sprayable coating based on polymer nanotubes. ACS Appl. Mater. Interfaces 2020, 12, 49694–49704. [Google Scholar] [CrossRef]
- Zeng, J.-L.; Zhu, F.-R.; Yu, S.-B.; Xiao, Z.-L.; Yan, W.-P.; Zheng, S.-H.; Zhang, L.; Sun, L.-X.; Cao, Z. Myristic acid/polyaniline composites as form stable phase change materials for thermal energy storage. Sol. Energy Mater. Sol. Cells 2013, 114, 136–140. [Google Scholar] [CrossRef]
- Zhu, Y.; Qin, Y.; Wei, C.; Liang, S.; Luo, X.; Wang, J.; Zhang, L. Nanoencapsulated phase change materials with polymer-SiO2 hybrid shell materials: Compo-sitions, morphologies, and properties. Energ. Convers. Manag. 2018, 164, 83–92. [Google Scholar] [CrossRef]
- Ji, W.; Cheng, X.; Chen, S.; Wang, X.; Li, Y. Self-assembly fabrication of GO/TiO2@paraffin microcapsules for enhancement of thermal energy storage. Powder Technol. 2021, 385, 546–556. [Google Scholar] [CrossRef]
- Jiang, X.; Luo, R.; Peng, F.; Fang, Y.; Akiyama, T.; Wang, S. Synthesis, characterization and thermal properties of paraffin microcapsules modified with nano-Al2O3. Appl. Energy 2015, 137, 731–737. [Google Scholar] [CrossRef]
- Sun, N.; Xiao, Z. Synthesis and performances of phase change materials microcapsules with a Polymer/BN/TiO2 hybrid shell for thermal energy storage. Energy Fuels 2017, 31, 10186–10195. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Su, J.; Zhang, X.; Wang, Y.; Tan, Y. Fabrication and characterization of novel electrothermal self-healing microcapsules with graphene/polymer hybrid shells for bitumenious material. Nanomaterials 2018, 8, 419. [Google Scholar] [CrossRef] [Green Version]
- Texter, J. Graphene oxide and graphene flakes as stabilizers and dispersing aids. Curr. Opin. Colloid Interface Sci. 2015, 20, 454–464. [Google Scholar] [CrossRef]
- Yu, Y.; He, L.; Lu, P.; Yuan, Y.; Zhang, H. Preparation, performances and applications of multi-functional photoluminescence form-stable phase change materials. Polymer 2020, 189, 122211. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Wu, D. Design and synthesis of multifunctional microencapsulated phase change materials with silver/silica double-layered shell for thermal energy storage, electrical conduction and antimicrobial effectiveness. Energy 2016, 111, 498–512. [Google Scholar] [CrossRef]
- Daou, I.; El-Kaddadi, L.; Zegaoui, O.; Asbik, M.; Zari, N. Structural, morphological and thermal properties of novel hybrid-microencapsulated phase change materials based on Fe2O3, ZnO and TiO2 nanoparticles for latent heat thermal energy storage applications. J. Energy Storage 2018, 17, 84–92. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, Y.; Sun, Y.; Sun, L.; Xu, F.; Zhang, J.; Zhou, H. A novel thermal-insulating film incorporating microencapsulated phase-change materials for temperature regulation and nano-TiO2 for UV-blocking. Sol. Energ. Mat. Sol. C. 2015, 137, 210–218. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Zhao, L.; Shi, X.; Song, G.; Tang, G. A facile approach to synthesize microencapsulated phase change materials embedded with silver nanoparticle for both thermal energy storage and antimicrobial purpose. Energy 2018, 158, 1052–1059. [Google Scholar] [CrossRef]
- Hou, P.; Qin, M.; Cui, S.; Zu, K. Preparation and characterization of metal-organic framework /microencapsulated phase change material composites for indoor hygrothermal control. J. Build. Eng. 2020, 31, 101345. [Google Scholar] [CrossRef]
- Zhao, Y.; Kong, W.; Jin, Z.; Fu, Y.; Wang, W.; Zhang, Y.; Liu, J.; Zhang, B. Storing solar energy within Ag-paraffin@halloysite microspheres as a novel self-heating catalyst. Appl. Energ. 2018, 222, 180–188. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, H.; Luo, J.; Liu, Y.; Song, G. Fabrication and properties of microencapsulated n-octadecane with TiO2 shell as thermal energy storage materials. Sol. Energy 2016, 127, 28–35. [Google Scholar] [CrossRef]
- Xu, B.; Zhou, J.; Ni, Z.; Zhang, C.; Lu, C. Synthesis of novel microencapsulated phase change materials with copper and copper oxide for solar energy storage and photo-thermal conversion. Sol. Energy Mater. Sol. Cells 2018, 179, 87–94. [Google Scholar] [CrossRef]
- Ma, X.; Liu, H.; Chen, C.; Liu, Y.; Zhang, L.; Xu, B.; Xiao, F. Synthesis of novel microencapsulated phase change material with SnO2/CNTs shell for solar energy storage and photo-thermal conversion. Mater. Res. Express 2020, 7, 015513. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, D. Tailoring of bifunctional microencapsulated phase change materials with CdS/SiO2 double-layered shell for solar photocatalysis and solar thermal energy storage. Appl. Therm. Eng. 2018, 134, 603–614. [Google Scholar] [CrossRef]
- Sun, W.; Wang, X.; Zhang, X.; Kong, X. Design and synthesis of microencapsulated phase-change materials with a poly(divinylbenzene)/dioxide titanium hybrid shell for energy storage and formaldehyde photodegradation. J. Phys. Chem. C 2020, 124, 20806–20815. [Google Scholar] [CrossRef]
- Cui, W.; He, J.; Wang, H.; Hu, J.; Liu, L.; Liang, Y. Polyaniline hybridization promotes photo-electro-catalytic removal of organic contaminants over 3D network structure of rGH-PANI/TiO2 hydrogel. Appl. Catal. B Environ. 2018, 232, 232–245. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semi-conductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Zheng, Z.; Xu, H.; Xu, Z.; Ge, J. A monodispersed spherical Zr-based metal-organic framework catalyst, Pt/Au@Pd@UIO-66, comprising an Au@Pd core-shell encapsulated in a UIO-66 center and its highly selective CO2 hydrogenation to produce CO. Small 2018, 14, 1702812. [Google Scholar] [CrossRef]
- Wang, G.; He, C.-T.; Huang, R.; Mao, J.; Wang, D.; Li, Y. Photoinduction of Cu single atoms decorated on UiO-66-NH2 for enhanced photocatalytic reduction of CO2 to liquid fuels. J. Am. Chem. Soc. 2020, 142, 19339–19345. [Google Scholar] [CrossRef]
- Ostad, M.I.; Shahrak, M.N.; Galli, F. Photocatalytic carbon dioxide reduction to methanol catalyzed by ZnO, Pt, Au, and Cu nanoparticles decorated zeolitic imidazolate framework-8. J. CO2 Util. 2021, 43, 101373. [Google Scholar] [CrossRef]
- Jin, J. Porphyrin-based metal–organic framework catalysts for photoreduction of CO2: Understanding the effect of node connectivity and linker metalation on activity. New J. Chem. 2020, 44, 15362–15368. [Google Scholar] [CrossRef]
- Duan, S.; Wu, S.; Wang, L.; She, H.; Huang, J.; Wang, Q. Rod-shaped metal organic framework structured PCN-222(Cu)/TiO2 composites for efficient photocatalytic CO2 reduction. Acta Phys. Chim. Sin. 2020, 36, 1905086. [Google Scholar] [CrossRef]
- Jin, J. Highly stable and efficient visible-light-driven carbon dioxide reduction by zirconium–metalloporphyrin PCN-222 via dual catalytic routes. React. Kinet. Mech. Catal. 2020, 131, 1–12. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, X.; Ma, Y.; Cho, H.S.; Ding, D.; Wang, C.; Wu, J.; Oleynikov, P.; Jia, M.; Cheng, J.; et al. Filling metal–organic framework mesopores with TiO2 for CO2 photoreduction. Nat. Cell Biol. 2021, 586, 549–554. [Google Scholar] [CrossRef]
- Cheng, X.M.; Gu, Y.; Zhang, X.Y.; Dao, X.Y.; Wang, S.Q.; Ma, J.; Zhao, J.; Sun, W.Y. Crystallographic facet heterojunction of MIL-125-NH2(Ti) for carbon dioxide photoreduction. Appl. Catal. B Environ. 2021, 298, 120524. [Google Scholar] [CrossRef]
- Hariri, R.; Saeed, D. Effective visible-light CO2 photoreduction over (metallo)porphyrin-based metal-organic frameworks to achieve useful hydrocarbons. Appl. Organomet. Chem. 2021, 35, 6422. [Google Scholar] [CrossRef]
- Xu, H.-Q.; Hu, J.; Wang, D.; Li, Z.; Zhang, Q.; Luo, Y.; Yu, S.-H.; Jiang, H.-L. Visible-light photoreduction of CO2 in a metal–organic framework: Boosting electron–hole separation via electron trap states. J. Am. Chem. Soc. 2015, 137, 13440–13443. [Google Scholar] [CrossRef] [PubMed]
- Pandiselvi, K.; Fang, H.; Huang, X.; Wang, J.; Xu, X.; Li, T. Constructing a novel carbon nitride/polyaniline/ZnO ternary heterostructure with enhanced photocatalytic performance using exfoliated carbon nitride nanosheets as supports. J. Hazard. Mater. 2016, 314, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, B.; Kumar, S.; Gil, J.M.; Pandiyan, V.; Aguiar, A.; Sobral, A.J.N. Highly active P25@Pd/C nanocomposite for the degradation of Naphthol Blue Black with visible light. J. Mol. Struct. 2018, 1153, 346–352. [Google Scholar] [CrossRef]
- Madaswamy, S.L.; Alfakeer, M.; Bahajjaj, A.A.A.; Ouladsmane, M.; Wabaidur, S.M.; Chen, C.-X.; Dhanusuraman, R. Remarkable electrocatalytic activity of Pd nanoparticles dispersed on polyaniline-polydiphenylamine copolymer nanocomposite for methanol and ethanol oxidation reaction. Synth. Met. 2021, 281, 116925. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Wang, J.; Sun, L.; Xie, T.; Yang, K.; Li, Z. Preparation and characterization of high efficiency microencapsulated phase change material based on paraffin wax core and SiO2 shell derived from sodium silicate precursor. Colloids Surfaces A 2021, 625, 126905. [Google Scholar] [CrossRef]
- Wei, C.; Zhou, H.; Liu, Q. PCN-222 MOF decorated conductive PEDOT films for sensitive electrochemical determination of chloramphenicol. Mater. Chem. Phys. 2021, 270, 124831. [Google Scholar] [CrossRef]
- Laabd, M.; Imgharn, A.; Hsini, A.; Naciri, Y.; Mobarak, M.; Szunerits, S.; Boukherroub, R.; Albourine, A. Efficient detoxification of Cr(VI)-containing effluents by sequential adsorption and reduction using a novel cysteine-doped PANi@faujasite composite: Experimental study supported by advanced statistical physics prediction. J. Hazard. Mater. 2022, 422, 126857. [Google Scholar] [CrossRef]
- Jiao, L.; Zhang, R.; Wan, G.; Yang, W.; Wan, X.; Zhou, H.; Shui, J.; Yu, S.-H.; Jiang, H.-L. Nanocasting SiO2 into metal–organic frameworks imparts dual protection to high-loading Fe single-atom electrocatalysts. Nat. Commun. 2020, 11, 2831. [Google Scholar] [CrossRef]
- Chai, L.; Wang, X.; Wu, D. Development of bifunctional microencapsulated phase change materials with crystalline titanium dioxide shell for latent-heat storage and photocatalytic effectiveness. Appl. Energy 2015, 138, 661–674. [Google Scholar] [CrossRef]
- Liu, J.; Fan, Y.; Zhang, K.; Zhang, L.; Su, K.-Y. Engineering porphyrin metal–organic framework composites as multifunctional platforms for CO2 adsorption and activation. J. Am. Chem. Soc. 2020, 142, 14548–14556. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, D. Fabrication of graphene/TiO2/paraffin composite phase change materials for enhancement of solar energy efficiency in photocatalysis and latent heat storage. ACS Sustain. Chem. Eng. 2017, 5, 4906–4915. [Google Scholar] [CrossRef]
- Zhao, Y.; Dong, Y.; Lu, F.; Ju, C.; Liu, L.; Zhang, J.; Zhang, B.; Feng, Y. Coordinative integration of a metal-porphyrinic framework and TiO2 nanoparticles for the formation of composite photocatalysts with enhanced visible-light-driven photocatalytic activities. J. Mater. Chem. A 2017, 5, 15380–15389. [Google Scholar] [CrossRef]
- Yuan, Y.P.; Yin, L.S.; Cao, S.W.; Xu, G.S.; Li, C.H.; Xue, C. Improving photocatalytic hydrogen production of metal-organic framework UiO-66 octahe-drons by dye-sensitization. Appl. Catal. B Environ. 2015, 168–169, 572–576. [Google Scholar] [CrossRef]
- Yao, K.; Liu, Y.; Yang, H.; Yuan, J.; Shan, S. Polyaniline-modified 3D-spongy SnS composites for the enhanced visible-light photocatalytic degradation of methyl orange. Colloids Surfaces A 2020, 603, 125240. [Google Scholar] [CrossRef]
- Wang, L.; Jin, P.; Huang, J.; She, H.; Wang, Q. Integration of copper(ii)-porphyrin zirconium metal–organic framework and titanium dioxide to construct Z-scheme system for highly improved photocatalytic CO2 reduction. ACS Sustain. Chem. Eng. 2019, 7, 15660–15670. [Google Scholar] [CrossRef]
- Feng, D.; Gu, Z.-Y.; Li, J.-R.; Jiang, H.-L.; Wei, Z.; Zhou, H. Zirconium-metalloporphyrin PCN-222: Mesoporous metal-organic frameworks with ultrahigh stability as biomimetic catalysts. Angew. Chem. Int. Ed. 2012, 51, 10307–10310. [Google Scholar] [CrossRef]
- Asano, N.; Uemura, S.; Kinugawa, T.; Akasaka, H.; Mizutani, T. Synthesis of biladienone and bilatrienone by coupled oxidation of tetraarylporphyrins. J. Org. Chem. 2007, 72, 5320–5326. [Google Scholar] [CrossRef]
- Park, J.; Jiang, Q.; Feng, D.; Mao, L.; Zhou, H.-C. Size-controlled synthesis of porphyrinic metal–organic framework and functionalization for targeted photodynamic therapy. J. Am. Chem. Soc. 2016, 138, 3518–3525. [Google Scholar] [CrossRef]
- Sami, S.; Etesami, N. Heat transfer enhancement of microencapsulated phase change material by addition of nanoparticles for a latent heat thermal energy storage system. Energy Rep. 2021, 7, 4930–4940. [Google Scholar] [CrossRef]
- Zhai, D.; He, Y.; Zhang, X.; Li, W. Preparation, Morphology, and thermal performance of microencapsulated phase change materials with a MF/SiO2 composite shell. Energy Fuels 2020, 34, 16819–16830. [Google Scholar] [CrossRef]
- Parvate, S.; Singh, J.; Dixit, P.; Vennapusa, J.R.; Maiti, T.K.; Chattopadhyay, S. Titanium dioxide nanoparticle-decorated polymer microcapsules enclosing phase change material for thermal energy storage and photocatalysis. ACS Appl. Polym. Mater. 2021, 3, 1866–1879. [Google Scholar] [CrossRef]
- Pornea, A.M.; Kim, H. Design and synthesis of SiO2/TiO2/PDA functionalized phase change microcapsules for efficient solar-driven energy storage. Energ. Convers. Manag. 2021, 232, 113801. [Google Scholar] [CrossRef]
- Wang, H.; Luo, J.; Yang, Y.; Zhao, L.; Song, G.; Tang, G. Fabrication and characterization of microcapsulated phase change materials with an additional function of thermochromic performance. Sol. Energy 2016, 139, 591–598. [Google Scholar] [CrossRef]
- Ruqaiya, A.N.; Guido, B.; Vladisavljevic, G.T. Microfluidic production of poly(1,6-hexanediol diacrylate)-based polymer microspheres and bifunctional microcapsules with embedded TiO2 nanoparticles. Langmuir 2018, 34, 11822–11831. [Google Scholar] [CrossRef] [Green Version]
- Jiang, F.; Wang, X.; Wu, D. Design and synthesis of magnetic microcapsules based on n-eicosane core and Fe3O4/SiO2 hybrid shell for dual-functional phase change materials. Appl. Energy 2014, 134, 456–468. [Google Scholar] [CrossRef]
- Sun, N.; Xiao, Z. Paraffin wax-based phase change microencapsulation embedded with silicon nitride nanoparticles for thermal energy storage. J. Mater. Sci. 2016, 51, 8550–8561. [Google Scholar] [CrossRef]
- Zhu, Y.; Chi, Y.; Liang, S.; Luo, X.; Chen, K.; Tian, C.; Wang, J.; Zhang, L. Novel metal coated nanoencapsulated phase change materials with high thermal conductivity for thermal energy storage. Sol. Energy Mater. Sol. Cells 2017, 176, 212–221. [Google Scholar] [CrossRef]
- Zhao, Q.; He, F.; Zhang, Q.; Fan, J.; He, R.; Zhang, K.; Yan, H.; Yang, W. Microencapsulated phase change materials based on graphene Pickering emulsion for light-to-thermal energy conversion and management. Sol. Energy Mater. Sol. Cells 2019, 203, 110204. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, W.; Hu, D.; Wu, L. Synthesis and characterization of microencapsulated methyl laurate with polyurethane shell materials via interfacial polymerization in Pickering emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124958. [Google Scholar] [CrossRef]
- Qiu, X.Z.; Tao, Y.; Xu, X.Q.; He, X.H.; Fu, X.Y. Synthesis and characterization of paraffin/TiO2-P(MMA-co-BA) phase change material microcapsules for thermal energy storage. J. Appl. Polym. Sci. 2018, 135, 46447. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, L.; Song, G.; Tang, G.; Shi, X. Organic-inorganic hybrid shell microencapsulated phase change materials prepared from SiO2/TiC-stabilized pickering emulsion polymerization. Sol. Energy Mater. Sol. Cells 2018, 175, 102–110. [Google Scholar] [CrossRef]
- Ji, X.; Chen, J.; Dang, G.; Zhang, Y.; Yang, J.; Li, W.; Zhao, Y.; Jin, Z. Preparation of phase change microcapsules with inorganic/polymer hybrid shell through a “two-step” reaction. Polym. Sci. Ser. B 2019, 61, 560–566. [Google Scholar] [CrossRef]
- Kuai, L.; Zhou, Y.; Tu, W.; Li, P.; Li, H.; Xu, Q.; Tang, L.; Wang, X.; Xiao, M.; Zou, Z. Rational construction of a CdS/reduced graphene oxide/TiO2 core–shell nanostructure as an all-solid-state Z-scheme system for CO2 photoreduction into solar fuels. RSC Adv. 2015, 5, 88409–88413. [Google Scholar] [CrossRef]
- Takayama, T.; Sato, K.; Fujimura, T.; Kojima, Y.; Iwase, A.; Kudo, A. Photocatalytic CO2 reduction using water as an electron donor by a powdered Z-scheme system consisting of metal sulfide and an RGO-TiO2 composite. Faraday Discuss. 2017, 198, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, X.; He, W.; Zhao, Y.; Wei, Y.; Xiong, J.; Liu, J.; Li, J.; Song, W.; Zhang, X.; et al. All-solid-state Z-scheme photocatalysts of g-C3N4/Pt/macroporous-(TiO2@carbon) for selective boosting visible-light-driven conversion of CO2 to CH4. J. Catal. 2020, 389, 440–449. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Y.; Xu, H.; Li, Y.; Wei, Y.; Liu, J.; Zhao, Z. Efficient Z-scheme photocatalysts of ultrathin g-C3N4-wrapped Au/TiO2-nanocrystals for enhanced visible-light-driven conversion of CO2 with H2O. Appl. Catal. B Environ. 2019, 263, 118314. [Google Scholar] [CrossRef]
- Raza, A.; Shen, H.; Haidry, A.A.; Sun, L.; Liu, R.; Cui, S. Studies of Z-scheme WO3-TiO2/Cu2ZnSnS4 ternary nanocomposite with enhanced CO2 photoreduction under visible light irradiation. J. CO2 Util. 2020, 37, 260–271. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, X.; Zhao, Y.; Wang, L.; Zhao, Z.; Huang, X.; Liu, J.; Li, J. Efficient photocatalysts of TiO2 nanocrystals-supported PtRu alloy nanoparticles for CO2 reduction with H2O: Synergistic effect of Pt-Ru. Appl. Catal. B Environ. 2018, 236, 445–457. [Google Scholar] [CrossRef]
- Rodríguez, V.; Camarillo, R.; Martínez, F.; Jiménez, C.; Rincón, J. CO2 photocatalytic reduction with CNT/TiO2 based nanocomposites prepared by high-pressure technology. J. Supercrit. Fluids 2020, 163, 104876. [Google Scholar] [CrossRef]
- Zhao, H.; Zheng, X.; Feng, X.; Li, Y. CO2 Reduction by plasmonic Au nanoparticle-decorated TiO2 photocatalyst with an ultrathin Al2O3 interlayer. J. Phys. Chem. C 2018, 122, 18949–18956. [Google Scholar] [CrossRef]









| Names | Melting Process | Crystallization Process | ΔT (°C) | Encapsulation Parameters | ||||
|---|---|---|---|---|---|---|---|---|
| Tm (°C) | ∆Hm (J·g−1) | Tc (°C) | ∆Hc (J·g−1) | Een (%) | Ees (%) | Ces (%) | ||
| Paraffin | 50.3 | 226.3 | 45.1 | 228.5 | 5.2 | - | - | - |
| PPTP-1 | 52.2 | 138.9 | 45.3 | 139.6 | 6.9 | 61.4 | 61.2 | 99.73 |
| PPTP-2 | 51.8 | 164.1 | 45.7 | 164.9 | 6.1 | 72.5 | 72.3 | 99.78 |
| PPTP-3 | 51.4 | 167.7 | 46.2 | 168.8 | 5.2 | 74.1 | 74.0 | 99.85 |
| PPTP-4 | 50.8 | 172.7 | 46.4 | 174.1 | 4.4 | 76.3 | 76.3 | 99.94 |
| PPTP-5 | 50.6 | 174.7 | 46.4 | 176.2 | 4.2 | 77.2 | 77.2 | 99.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Hou, Y.; Zhang, X. Bi-Functional Paraffin@Polyaniline/TiO2/PCN-222(Fe) Microcapsules for Solar Thermal Energy Storage and CO2 Photoreduction. Nanomaterials 2022, 12, 2. https://doi.org/10.3390/nano12010002
Sun W, Hou Y, Zhang X. Bi-Functional Paraffin@Polyaniline/TiO2/PCN-222(Fe) Microcapsules for Solar Thermal Energy Storage and CO2 Photoreduction. Nanomaterials. 2022; 12(1):2. https://doi.org/10.3390/nano12010002
Chicago/Turabian StyleSun, Wenchang, Yueming Hou, and Xu Zhang. 2022. "Bi-Functional Paraffin@Polyaniline/TiO2/PCN-222(Fe) Microcapsules for Solar Thermal Energy Storage and CO2 Photoreduction" Nanomaterials 12, no. 1: 2. https://doi.org/10.3390/nano12010002