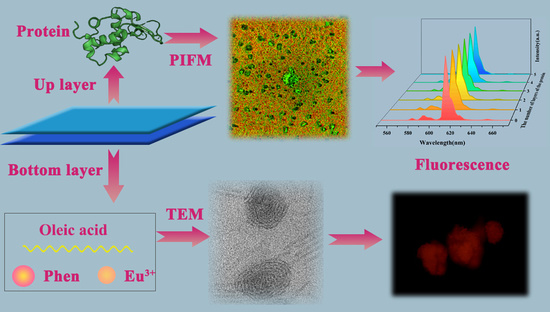
Stable Fluorescence of Eu3+ Complex Nanostructures Beneath a Protein Skin for Potential Biometric Recognition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Section
2.3. Characterization
3. Results
3.1. Fingerprint-Like Nanostructures Characterized by TEM, HRTEM, and EDS Mapping
3.2. UV–Visible Absorption Spectra Analysis
3.3. Fluorescence Properties of OEu3+Phen Complexes
3.4. Observing Luminescent Nanostructures under a Protein Skin Layer by PiFM
3.5. The Effect of Different Thicknesses of Protein Layers
3.6. Prognosis for Biometric Recognition Applications
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, V. Implantable RFID Chips; Springer: Boston, MA, USA, 2008. [Google Scholar]
- Masters, A.; Michael, K. Lend me your arms: The use and implications of humancentric RFID. Electron. Commer. Res. Appl. 2007, 6, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Sancho-Fornes, G.; Avella-Oliver, M.; Carrascosa, J.; Puchades, R.; Maquieira, Á. Interferometric multilayered nanomaterials for imaging unlabeled biorecognition events. Sens. Actuators B Chem. 2021, 331, 129289. [Google Scholar] [CrossRef]
- Langyan, R.; Chauhan, A.; Lohra, S.; Dhania, S.L. Judd-Ofelt analysis of some novel Eu3+ complexes featuring Kojic acid and N, N’- donor ligands. J. Photochem. Photobiol. A Chem. 2020, 401, 112752. [Google Scholar] [CrossRef]
- Song, H.; Fan, C.; Wang, R.; Wang, Z.; Pu, S. 1,10-Phenanthroline decorated with substituent groups forming europium(III) complexes: Synthesis, crystal structure, photoluminescence properties and their bioimaging in living cells. J. Coord. Chem. 2020, 73, 2311–2327. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, A.; Wu, Y.; Gao, Z.; Xie, H. Synthesis and luminescent properties of novel red-emitting Eu(III) complexes based on alanine aliphatic derivatives with different optical rotation. Mater. Res. Express 2020, 7, 025102. [Google Scholar] [CrossRef]
- Santos, J.A.O.; Mutti, A.M.G.; Bispo, A.G., Jr.; Pires, A.M.; Lima, S.A.M. Red-Emitting Hybrid Based on Eu3+-dbm Complex Anchored on Silica Nanoparticles Surface by Carboxylic Acid for Biomarker Application. Materials 2020, 13, 5494. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Deng, Q.; Yang, S.; Guo, D. Preparation and fluorescence properties of novel 2-quinolone derivatives and their corresponding Eu(III) complexes. Colloids Surf. A Physicochem. Eng. Asp. 2020, 599, 124861. [Google Scholar] [CrossRef]
- Lu, P.; Wang, Y.; Huang, L.; Lian, S.; Kipper, M.J. Tb3+/Eu3+ Complex-Doped Rigid Nanoparticles in Transparent Nanofibrous Membranes Exhibit High Quantum Yield Fluorescence. Nanomaterials 2020, 10, 694. [Google Scholar] [CrossRef] [Green Version]
- Shavaleev, N.M.; Gumy, F.; Scopelliti, R.; Bunzli, J.-C. Highly Luminescent Homoleptic Europium Chelates. Inorg. Chem. 2009, 48, 5611–5613. [Google Scholar] [CrossRef]
- Maynard, B.A.; Smith, P.A.; Ladner, L.; Jaleel, A.; Beedoe, N.; Crawford, C.; Assefa, Z.; Sykora, R.E. Emission enhancement through dual donor sensitization: Modulation of structural and spectroscopic properties in a series of europium tetracyanoplatinates. Inorg. Chem. 2009, 48, 6425–6435. [Google Scholar] [CrossRef]
- Schnaars, K.; Kaneko, M.; Fujisawa, K. Effect of Oxygen-Donor Charge on Adjacent Nitrogen-Donor Interactions in Eu3+ Complexes of Mixed N,O-Donor Ligands Demonstrated on a 10-Fold [Eu(TPAMEN)]3+ Chelate Complex. Inorg. Chem. 2021, 60, 2477–2491. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Chen, W.; Ding, Y. Facile synthesis and characterization of macromolecular Eu(III) complexes with β-diketone ligands and poly(4-vinyl pyridine-co-methyl methacrylate). J. Polym. Res. 2020, 27, 1–8. [Google Scholar] [CrossRef]
- Ye, H.R.; Dong, X.Y.; Xu, M.; Cheng, X.; Dai, J.G.; Zhang, J. Research Progress of Europium Complexes Luminescent Materials. Mater. Sci. Forum 2020, 1001, 1–15. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, L.; Zhang, H.; Zheng, Y.; Li, H.; Deng, R.; Peng, Z.; Li, Z. Efficient Electroluminescence from New Lanthanide (Eu3+, Sm3+) Complexes. Inorg. Chem. 2005, 44, 1611–1618. [Google Scholar] [CrossRef]
- Kalinovskaya, I.V. Spectral-Luminescent Properties of Mixed-Ligand Europium(III) Complexes with O-Methoxybenzoic Acid and Phosphorus-Containing Neutral Ligands. Opt. Spectrosc. 2020, 128, 698–701. [Google Scholar] [CrossRef]
- Singh, D.; Bhagwan, S.; Dalal, A.; Nehra, K.; Saini, R.K.; Singh, K.; Simantilleke, A.P.; Kumar, S.; Singh, I. Oxide ancillary ligand-based europium β-diketonate complexes and their enhanced luminosity. Rare Met. 2021, 40, 2873–2881. [Google Scholar] [CrossRef]
- Moriguchi, T.; Kawata, H.; Jalli, V. Design, Synthesis, Crystal Structure and Photoluminescence Properties of Four New Europium (III) Complexes with Fluorinated β-Diketone Ligand. Cryst. Struct. Theory Appl. 2021, 10, 1–13. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, X. Synthesis, Crystal Structure, and Visible Light Sensitized Red Luminescence of a Novel Salen-Bridged Pentanuclear Eu-Na Complex. Russ. J. Inorg. Chem. 2020, 65, 1166–1172. [Google Scholar] [CrossRef]
- Song, H.; Liu, G.; Fan, C.; Pu, S. A novel fluorescent sensor for Al3+ and Zn2+ based on a new europium complex with a 1,10-phenanthroline ligand. J. Rare Earths 2021, 39, 460–468. [Google Scholar] [CrossRef]
- De-Hui, S.; Ji-Lin, Z.; Yan-Lin, L.; Guang-Yan, H.; Jia-Zuan, N. Synthesis and Characterization of [Eu(DBM)3phen]Cl3@SiO2–NH2 Composite Nanoparticles. J. Nanosci. Nanotechnol. 2011, 11, 9656–9660. [Google Scholar] [CrossRef]
- Amao, Y.; Okura, I.; Miyashita, T. Thenoyltrifluoroacetonato 1,10-Phenanthroline Europium (III) Complex Immobilized in Fluoropolymer Film as Optical Oxygen Sensing Material. Chem. Lett. 2000, 78, 934–935. [Google Scholar] [CrossRef]
- Shahi, P.K.; Singh, A.; Singh, S.K.; Rai, S.B.; Ullrich, B. Revelation of the Technological Versatility of the Eu(TTA)3Phen Complex by Demonstrating Energy Harvesting, Ultraviolet Light Detection, Temperature Sensing, and Laser Applications. ACS Appl. Mater. Interfaces 2015, 7, 18231–18239. [Google Scholar] [CrossRef] [PubMed]
- Ishizaka, T.; Kasai, H.; Nakanishi, H. Fabrication and luminescence properties of Eu-complex/polyimide composite nanoparticles. J. Mater. Sci. 2009, 44, 166–169. [Google Scholar] [CrossRef]
- Ye, J.; Wang, B.; Xiong, J.; Sun, R. Enhanced fluorescence and structural characteristics of carboxymethyl cellulose/Eu(III) nano-complex: Influence of reaction time. Carbohydr. Polym. 2016, 135, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tang, J.; Wang, G.; Wang, W.; Ren, J.; Ding, W.; Zhang, X.; Wang, Y.; Shen, W.; Huang, L.; et al. Ln3+-Induced Diblock Copolymeric Aggregates for Fully Flexible Tunable White-Light Materials. Nanomaterials 2019, 9, 363. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Sun, L.-D.; Yan, C.-H. Luminescent rare earth nanomaterials for bioprobe applications. Dalton Trans. 2008, 5687–5697. [Google Scholar] [CrossRef]
- Wen, Q.; Liu, W.; Zheng, Y.; Gao, J. Realization of Optical Network Structures for Robust Films through Immobilization of Europium Complexes. J. Fluoresc. 2019, 29, 1285–1290. [Google Scholar] [CrossRef]
- Ma, H.; Chen, K.; Song, B.; Tang, Z.; Huang, Y.; Zhang, T.; Wang, H.; Sun, W.; Yuan, J. A visible-light-excitable mitochondria-targeted europium complex probe for hypochlorous acid and its application to time-gated luminescence bioimaging. Biosens. Bioelectron. 2020, 168, 112560. [Google Scholar] [CrossRef]
- Binnemans, K. ChemInform Abstract: Lanthanide-Based Luminescent Hybrid Materials. Cheminform 2009, 40. [Google Scholar] [CrossRef]
- Dandekar, M.P.; Itankar, S.G.; Nandanwar, D.V.; Kondawar, S.B. Electrospun Eu (TTA)3 Phen/Polymer Blend Nanofibers for Photoluminescent Smart Fabrics. In NAC 2019; Springer: Berlin/Heidelberg, Germany, 2020; pp. 95–105. [Google Scholar]
- Xu, Q.; Tang, J.; Wang, Y.; Liu, J.; Wang, X.; Huang, Z.; Huang, L.; Wang, Y.; Shen, W.; Belfiore, L.A. Eu3+-induced aggregates of diblock copolymers and their photoluminescent property. J. Colloid Interface Sci. 2013, 394, 630. [Google Scholar] [CrossRef]
- Ruan, L.; Gao, X.; Zhao, J.; Xu, C.; Liang, D. Preparation and characteristics of Eu(DBM)3phen: Synthesis, single-crystal structure and spectroscopic analysis. J. Mol. Struct. 2017, 1149, 265–272. [Google Scholar] [CrossRef]
- Rimal, V.; Shishodia, S.; Srivastava, P.K. Novel synthesis of high-thermal stability carbon dots and nanocomposites from oleic acid as an organic substrate. Appl. Nanosci. 2020, 10, 455–464. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Y.; Wang, B.; Su, Z. Synthesis and fluorescent properties of europium–polymer complexes containing 1,10-phenanthroline. Synth. Met. 2009, 159, 1557–1562. [Google Scholar] [CrossRef]
- Guo, X.; Fu, L.; Zhang, H.; Carlos, L.D.; Peng, C.; Guo, J.; Yu, J.; Deng, R.; Sun, L. Incorporation of luminescent lanthanide complex inside the channels of organically modified mesoporous silica via template-ion exchange method. New J. Chem. 2005, 29, 1351–1358. [Google Scholar] [CrossRef]
- Shang, M.; Li, C.; Lin, J. How to produce white light in a single-phase host? Chem. Soc. Rev. 2014, 43, 1372–1386. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Thirumalai, J.; Thomas, S.; Gowri, M. Luminescence and magnetic behaviour of almond like (Na0.5La0.5)MoO4:RE3+ (RE=Eu, Tb, Dy) nanostructures. J. Alloy. Compd. 2014, 604, 20–30. [Google Scholar] [CrossRef]
- Devi, R.; Vaidyanathan, S. Narrow band red emitting europium complexes and their application in smart white LEDs and vapoluminescent sensors. Dalton Trans. 2020, 49, 6205–6219. [Google Scholar] [CrossRef] [PubMed]
- Lewis, W.K.; Rosenberger, A.T.; Gord, J.R.; Crouse, C.A.; Harruff, B.A.; Fernando, K.S.; Smith, M.J.; Phelps, D.K.; Spowart, J.E.; Guliants, E.A. Multispectroscopic (FTIR, XPS, and TOFMS− TPD) investigation of the core− shell bonding in sonochemically prepared aluminum nanoparticles capped with oleic acid. J. Phys. Chem. C 2010, 114, 6377–6380. [Google Scholar] [CrossRef]
- Reinhardt, L.; Sacksteder, K.A.; Cleland, W.W. Enthalpic Studies of Complex Formation between Carboxylic Acids and 1-Alkylimidazoles. J. Am. Chem. Soc. 1998, 120, 13366–13369. [Google Scholar] [CrossRef]
- Harris, R.A.; Shumbula, P.M.; Van Der Walt, H. Analysis of the Interaction of Surfactants Oleic Acid and Oleylamine with Iron Oxide Nanoparticles through Molecular Mechanics Modeling. Langmuir 2015, 31, 3934–3943. [Google Scholar] [CrossRef]
- Jiang, L.; Zheng, J.-W.; Chen, W.-C.; Ye, J.-J.; Mo, L.-E.; Li, Z.; Hu, L.-H.; Zhang, C.-N.; Dai, S.-Y. Tuning Coordination Environment: Better Photophysical Performance of Europium(III) Complex. J. Phys. Chem. C 2017, 121, 5925–5930. [Google Scholar] [CrossRef]
- Murdick, R.A.; Morrison, W.; Nowak, D.; Albrecht, T.R.; Jahng, J.; Park, S. Photoinduced force microscopy: A technique for hyperspectral nanochemical mapping. Jpn. J. Appl. Phys. 2017, 56, 08LA04. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.-G.; Yang, Y.; Yao, J.; Xue, L.; Chen, S.; Li, X.; Morrison, W.; Yang, C.; Li, Y. Constructing a Strongly Absorbing Low-Bandgap Polymer Acceptor for High-Performance All-Polymer Solar Cells. Angew. Chem. Int. Ed. 2017, 56, 13503–13507. [Google Scholar] [CrossRef]
- Zhang, H.; Song, H.; Dong, B.; Han, L.; Pan, G.; Bai, X.; Fan, L.; Lu, S.; Zhao, H.; Wang, F. Electrospinning Preparation and Luminescence Properties of Europium Complex/Polymer Composite Fibers. J. Phys. Chem. C 2008, 112, 9155–9162. [Google Scholar] [CrossRef]
- Wan, C.; Li, M.; Bai, X.; Zhang, Y. Synthesis and Characterization of Photoluminescent Eu(III) Coordination Halloysite Nanotube-Based Nanohybrids. J. Phys. Chem. C 2009, 113, 16238–16246. [Google Scholar] [CrossRef]






| Height/nm | Height of Protein/nm | |
|---|---|---|
| OEu3+Phen complex layer | 20 | |
| One layer of protein skin layer | 361 | 341 |
| Two layers of protein skin layer | 665 | 645 |
| Three layers of protein skin layer | 946 | 926 |
| Four layers of protein skin layer | 1256 | 1236 |
| Five layers of protein skin layer | 1584 | 1564 |
| τ1/ms | τ2/ms | B1 | B2 | Lifetime/ms | Quantum Yield |
|---|---|---|---|---|---|
| 0.6708 | 0.8254 | 20.26% | 79.74% | 0.79408 | 64.17% |
| 0.7444 | 0.8097 | 24.59% | 75.41% | 0.79365 | 66.32% |
| 0.6654 | 0.8393 | 29% | 71% | 0.78886 | 66.96% |
| 0.6924 | 0.8115 | 19.26% | 80.74% | 0.78857 | 67.09% |
| 0.5617 | 0.8596 | 24.01% | 75.99% | 0.78808 | 67.30% |
| 0.6283 | 0.8282 | 20% | 80% | 0.78822 | 68.01% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Yao, Z.; Snow, C.D.; Xu, Y.; Wang, Y.; Xiu, D.; Belfiore, L.A.; Tang, J. Stable Fluorescence of Eu3+ Complex Nanostructures Beneath a Protein Skin for Potential Biometric Recognition. Nanomaterials 2021, 11, 2462. https://doi.org/10.3390/nano11092462
Zhao Y, Yao Z, Snow CD, Xu Y, Wang Y, Xiu D, Belfiore LA, Tang J. Stable Fluorescence of Eu3+ Complex Nanostructures Beneath a Protein Skin for Potential Biometric Recognition. Nanomaterials. 2021; 11(9):2462. https://doi.org/10.3390/nano11092462
Chicago/Turabian StyleZhao, Yue, Ziyu Yao, Christopher D. Snow, Yanan Xu, Yao Wang, Dan Xiu, Laurence A. Belfiore, and Jianguo Tang. 2021. "Stable Fluorescence of Eu3+ Complex Nanostructures Beneath a Protein Skin for Potential Biometric Recognition" Nanomaterials 11, no. 9: 2462. https://doi.org/10.3390/nano11092462