High-Throughput Calculations on the Decomposition Reactions of Off-Stoichiometry GeSbTe Alloys for Embedded Memories
Abstract
:1. Introduction
2. Computational Details
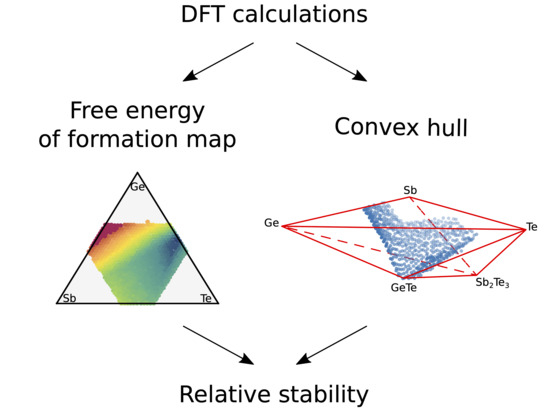
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GST | GeSbTe |
| PCMs | phase change memories |
| GSTXYZ | GeSbYe |
| DFT | Density Functional Theory |
| PBE | Perdew, Burke and Ernzerhof |
| vdW | van der Waals |
| BZ | Brillouin zone |
| SQS | Special quasi-random structure |
Appendix A

References
- Wuttig, M.; Yamada, N. Phase-change materials for rewriteable data storage. Nat. Mater. 2007, 6, 824–832. [Google Scholar] [CrossRef]
- Noé, P.; Vallée, C.; Hippert, F.; Fillot, F.; Raty, J.Y. Phase-change materials for non-volatile memory devices: From technological challenges to materials science issues. Semicond. Sci. Technol. 2018, 33, 013002. [Google Scholar] [CrossRef]
- Zhang, W.; Mazzarello, R.; Wuttig, M.; Ma, E. Designing crystallization in phase-change materials for universal memory and neuro-inspired computing. Nat. Rev. Mater. 2019, 4, 150–168. [Google Scholar] [CrossRef]
- Pirovano, A.; Lacaita, A.; Benvenuti, A.; Pellizzer, F.; Bez, R. Electronic switching in phase-change memories. IEEE Trans. Electron Devices 2004, 51, 452–459. [Google Scholar] [CrossRef]
- Boybat, I.; Le Gallo, M.; Nandakumar, S.R.; Moraitis, T.; Parnell, T.; Tuma, T.; Rajendran, B.; Leblebici, Y.; Sebastian, A.; Eleftheriou, E. Neuromorphic computing with multi-memristive synapses. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Sebastian, A.; Le Gallo, M.; Khaddam-Aljameh, R.; Eleftheriou, E. Memory devices and applications for in-memory computing. Nat. Nanotechnol. 2020, 15, 529–544. [Google Scholar] [CrossRef]
- Satoh, I.; Yamada, N. DVD-RAM for all audio/video, PC, and network applications. In Fifth International Symposium on Optical Storage (ISOS 2000); Gan, F., Hou, L., Eds.; International Society for Optics and Photonics, SPIE: Bellingham, WA, USA, 2001; Volume 4085, pp. 283–290. [Google Scholar] [CrossRef]
- Feldmann, J.; Youngblood, N.; Karpov, M.; Gehring, H.; Li, X.; Stappers, M.; Le Gallo, M.; Fu, X.; Lukashchuk, A.; Raja, A.S.; et al. Parallel convolutional processing using an integrated photonic tensor core. Nature 2021, 589, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Wuttig, M.; Bhaskaran, H.; Taubner, T. Phase-change materials for non-volatile photonic applications. Nat. Photon. 2017, 11, 465–476. [Google Scholar] [CrossRef]
- Adler, D.; Shur, M.; Silver, M.; Ovshinsky, S. Threshold switching in chalcogenide-glass thin films. J. Appl. Phys. 1980, 51, 3289–3309. [Google Scholar] [CrossRef]
- Raoux, S.; Wełnic, W.; Ielmini, D. Phase Change Materials and Their Application to Nonvolatile Memories. Chem. Rev. 2010, 110, 240–267. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.L.; Sebastian, A. An overview of phase-change memory device physics. J. Phys. D Appl. Phys. 2020, 53, 213002. [Google Scholar] [CrossRef]
- Pries, J.; Wei, S.; Wuttig, M.; Lucas, P. Switching between Crystallization from the Glassy and the Undercooled Liquid Phase in Phase Change Material Ge2Sb2Te5. Adv. Mater. 2019, 31, 1900784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Ma, E. Unveiling the structural origin to control resistance drift in phase-change memory materials. Mater. Today 2020, 41, 156–176. [Google Scholar] [CrossRef]
- Choe, J. Intel 3D XPoint Memory Die Removed from Intel Optane™ PCM (Phase Change Memory). 2017. Available online: https://www.techinsights.com/blog/intel-3d-xpoint-memory-die-removed-intel-optanetm-pcm-phase-change-memory (accessed on 7 August 2021).
- Cappelletti, P.; Annunziata, R.; Arnaud, F.; Disegni, F.; Maurelli, A.; Zuliani, P. Phase change memory for automotive grade embedded NVM applications. J. Phys. D Appl. Phys. 2020, 53, 193002. [Google Scholar] [CrossRef]
- Zuliani, P.; Palumbo, E.; Borghi, M.; Dalla Libera, G.; Annunziata, R. Engineering of chalcogenide materials for embedded applications of Phase Change Memory. Solid State Electron. 2015, 111, 27–31. [Google Scholar] [CrossRef]
- Palumbo, E.; Zuliani, P.; Borghi, M.; Annunziata, R. Forming operation in Ge-rich GexSbyTez phase change memories. Solid State Electron. 2017, 133, 38–44. [Google Scholar] [CrossRef]
- Noé, P.; Sabbione, C.; Bernier, N.; Castellani, N.; Fillot, F.; Hippert, F. Impact of interfaces on scenario of crystallization of phase change materials. Acta Mater. 2016, 110, 142–148. [Google Scholar] [CrossRef]
- Navarro, G.; Coué, M.; Kiouseloglou, A.; Noé, P.; Fillot, F.; Delaye, V.; Persico, A.; Roule, A.; Bernard, M.; Sabbione, C.; et al. Trade-off between SET and data retention performance thanks to innovative materials for phase-change memory. In Proceedings of the 2013 IEEE International Electron Devices Meeting, Washington, DC, USA, 9–11 December 2013; pp. 21.5.1–21.5.4. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Hsu, T.H.; Raoux, S.; Wu, J.; Du, P.Y.; Breitwisch, M.; Zhu, Y.; Lai, E.K.; Joseph, E.; Mittal, S.; et al. A high performance phase change memory with fast switching speed and high temperature retention by engineering the GexSbyTez phase change material. In Proceedings of the 2011 International Electron Devices Meeting, Washington, DC, USA, 5–7 December 2011; pp. 3.4.1–3.4.4. [Google Scholar] [CrossRef]
- Agati, M.; Vallet, M.; Joulié, S.; Benoit, D.; Claverie, A. Chemical phase segregation during the crystallization of Ge-rich GeSbTe alloys. J. Mater. Chem. C 2019, 7, 8720–8729. [Google Scholar] [CrossRef]
- Privitera, S.M.S.; Sousa, V.; Bongiorno, C.; Navarro, G.; Sabbione, C.; Carria, E.; Rimini, E. Atomic diffusion in laser irradiated Ge rich GeSbTe thin films for phase change memory applications. J. Phys. D Appl. Phys. 2018, 51, 145103. [Google Scholar] [CrossRef]
- Privitera, S.M.; López García, I.; Bongiorno, C.; Sousa, V.; Cyrille, M.C.; Navarro, G.; Sabbione, C.; Carria, E.; Rimini, E. Crystallization properties of melt-quenched Ge-rich GeSbTe thin films for phase change memory applications. J. Appl. Phys. 2020, 128, 155105. [Google Scholar] [CrossRef]
- Henry, L.; Bernier, N.; Jacob, M.; Navarro, G.; Clément, L.; Rouvière, J.L.; Robin, E. Studying phase change memory devices by coupling scanning precession electron diffraction and energy dispersive X-ray analysis. Acta Mater. 2020, 201, 72–78. [Google Scholar] [CrossRef]
- Luong, M.; Agati, M.; Ramond, N.; Grisolia, J.; Le Friec, Y.; Benoit, D.; Claverie, A. On Some Unique Specificities of Ge-Rich GeSbTe Phase- Change Material Alloys for Nonvolatile Embedded-Memory Applications. Phys. Status Solidi RRL 2021, 15, 2000471. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, Y.X.; Wang, X.D.; Chen, Y.H.; Deringer, V.L.; Mazzarello, R.; Zhang, W. Ab initio molecular dynamics and materials design for embedded phase-change memory. npj Comput. Mater. 2021, 7, 29. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goedecker, S.; Teter, M. Separable dual-space Gaussian pseudopotentials. Phys. Rev. B Condens. Matter 1996, 54, 1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krack, M. Pseudopotentials for H to Kr optimized for gradient-corrected exchange-correlation functionals. Theor. Chem. Acc. 2005, 114, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Vandevondele, J.; Krack, M.; Mohamed, F.; Parrinello, M.; Chassaing, T.; Hutter, J. Quickstep: Fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput. Phys. Commun. 2005, 167, 103–128. [Google Scholar] [CrossRef] [Green Version]
- Weber, H.; Schumacher, M.; Jóvári, P.; Tsuchiya, Y.; Skrotzki, W.; Mazzarello, R.; Kaban, I. Experimental and ab initio molecular dynamics study of the structure and physical properties of liquid GeTe. Phys. Rev. B 2017, 96, 054204. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, M.; Weber, H.; Jóvári, P.; Tsuchiya, Y.; Youngs, T.G.; Kaban, I.; Mazzarello, R. Structural, electronic and kinetic properties of the phase-change material Ge2Sb2Te5 in the liquid state. Sci. Rep. 2016, 6, 27434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Wuttig, M. The Dependence of Crystal Structure of Te-Based Phase-Change Materials on the Number of Valence Electrons. Adv. Mater. 2004, 16, 439–443. [Google Scholar] [CrossRef]
- Zunger, A.; Wei, S.H.; Ferreira, L.G.; Bernard, J.E. Special quasirandom structures. Phys. Rev. Lett. 1990, 65, 353. [Google Scholar] [CrossRef] [Green Version]
- Van De Walle, A.; Tiwary, P.; De Jong, M.; Olmsted, D.L.; Asta, M.; Dick, A.; Shin, D.; Wang, Y.; Chen, L.Q.; Liu, Z.K. Efficient stochastic generation of special quasirandom structures. Calphad 2013, 42, 13–18. [Google Scholar] [CrossRef]
- Adenis, C.; Langer, V.; Lindqvist, O. Reinvestigation of the structure of tellurium. Acta Cryst. C 1989, 45, 941–942. [Google Scholar] [CrossRef]
- Barrett, C.S.; Cucka, P.; Haefner, K. The crystal structure of antimony at 4.2, 78 and 298 K. Acta Cryst. 1963, 16, 451–453. [Google Scholar] [CrossRef]
- Goldak, J.; Barrett, C.S.; Innes, D.; Youdelis, W. Structure of α-GeTe. J. Chem. Phys 1966, 44, 3323–3325. [Google Scholar] [CrossRef]
- Anderson, T.L.; Krause, H.B. Refinement of the Sb2Te3 and Sb2Te2Se structures and their relationship to nonstoichiometric Sb2Te3-ySey compounds. Acta Cryst. B 1974, 30, 1307–1310. [Google Scholar] [CrossRef] [Green Version]
- Abou El Kheir, O.; Dragoni, D.; Bernasconi, M. Density functional simulations of decomposition pathways of Ge-rich GeSbTe alloys for phase change memories. Phys. Rev. Mat. 2021, in press. [Google Scholar]
- Giessen, B.; Borromee-Gautier, C. Structure and alloy chemistry of metastable GeSb. J. Solid State Chem. 1972, 4, 447–452. [Google Scholar] [CrossRef]
- Li, K.; Peng, L.; Zhu, L.; Zhou, J.; Sun, Z. Vacancy-mediated electronic localization and phase transition in cubic Sb2Te3. Mater. Sci. Semicond. Process. 2021, 135, 106052. [Google Scholar] [CrossRef]
- Hildebrandt, G.; Glasser, D. Predicting phase and chemical equilibrium using the convex hull of the Gibbs free energy. Chem. Eng. J. 1994, 54, 187–197. [Google Scholar] [CrossRef]
- Curtarolo, S.; Morgan, D.; Ceder, G. Accuracy of ab initio methods in predicting the crystal structures of metals: A review of 80 binary alloys. Comput. Coupling Phase Diagrams Thermochem. 2005, 29, 163–211. [Google Scholar] [CrossRef] [Green Version]
- Nyshadham, C.; Oses, C.; Hansen, J.E.; Takeuchi, I.; Curtarolo, S.; Hart, G.L.W. A computational high-throughput search for new ternary superalloys. Acta Mater. 2017, 122, 483–487. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, T.; Kojima, R.; Yamada, N.; Kifune, K.; Kubota, Y.; Takata, M. Structural Features of Ge1Sb4Te7, an Intermetallic Compound in the GeTe-Sb2Te3 Homologous Series. Chem. Mater. 2008, 20, 5750–5755. [Google Scholar] [CrossRef]
- Matsunaga, T.; Yamada, N.; Kubota, Y. Structures of stable and metastable Ge2Sb2Te5, an intermetallic compound in GeTe–Sb2Te3 pseudobinary systems. Acta Cryst. B 2004, 60, 685–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsunaga, T.; Morita, H.; Kojima, R.; Yamada, N.; Kifune, K.; Kubota, Y.; Tabata, Y.; Kim, J.J.; Kobata, M.; Ikenaga, E.; et al. Structural characteristics of GeTe-rich GeTe–Sb2Te3 pseudobinary metastable crystals. J. Appl. Phys. 2008, 103, 093511. [Google Scholar] [CrossRef]
- Govaerts, K.; Sluiter, M.H.; Partoens, B.; Lamoen, D. Stability of Sb-Te layered structures: First-principles study. Phys. Rev. B Condens. Matter 2012, 85, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Barber, C.B.; Dobkin, D.P.; Huhdanpaa, H. The Quickhull algorithm for convex hulls. ACM Trans. Math. Softw. 1996, 22, 469–483. [Google Scholar] [CrossRef] [Green Version]
- Evang, V.; Mazzarello, R. Point defects in disordered and stable GeSbTe phase-change materials. Mater. Sci. Semicond. Process. 2021, 133, 105948. [Google Scholar] [CrossRef]
- Caravati, S.; Bernasconi, M.; Kühne, T.D.; Krack, M.; Parrinello, M. Coexistence of tetrahedral- and octahedral-like sites in amorphous phase change materials. Appl. Phys. Lett. 2007, 91, 171906. [Google Scholar] [CrossRef]
- Caravati, S.; Bernasconi, M.; Kühne, T.D.; Krack, M.; Parrinello, M. First-principles study of crystalline and amorphous Ge2Sb2Te5 and the effects of stoichiometric defects. J. Condens. Matter Phys. 2009, 21, 255501. [Google Scholar] [CrossRef]
- Gabardi, S.; Caravati, S.; Bernasconi, M.; Parrinello, M. Density functional simulations of Sb-rich GeSbTe phase change alloys. J. Condens. Matter Phys. 2012, 24, 385803. [Google Scholar] [CrossRef]
- Sosso, G.C.; Caravati, S.; Mazzarello, R.; Bernasconi, M. Raman spectra of cubic and amorphous Ge2Sb2Te5 from first principles. Phys. Rev. B 2011, 83, 134201. [Google Scholar] [CrossRef]
- Akola, J.; Jones, R.O. Structural phase transitions on the nanoscale: The crucial pattern in the phase-change materials Ge2Sb2Te5 and GeTe. Phys. Rev. B 2007, 76, 235201. [Google Scholar] [CrossRef] [Green Version]
- Hegedüs, J.; Elliott, S.R. Microscopic origin of the fast crystallization ability of Ge–Sb–Te phase-change memory materials. Nat. Mater. 2008, 7, 399–405. [Google Scholar] [CrossRef]
- Kalikka, J.; Akola, J.; Jones, R.O. Crystallization processes in the phase change material Ge2Sb2Te5: Unbiased density functional/molecular dynamics simulations. Phys. Rev. B 2016, 94, 134105. [Google Scholar] [CrossRef] [Green Version]
- Sosso, G.C.; Miceli, G.; Caravati, S.; Giberti, F.; Behler, J.; Bernasconi, M. Fast Crystallization of the Phase Change Compound GeTe by Large-Scale Molecular Dynamics Simulations. J. Phys. Chem. Lett. 2013, 4, 4241–4246. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Wang, J.; Zhou, Y.; Tian, H.; Lu, L.; Mazzarello, R.; Jia, C.; Zhang, W.; Rao, F.; Ma, E. Phase-change heterostructure enables ultralow noise and drift for memory operation. Science 2019, 366, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Behler, J. First Principles Neural Network Potentials for Reactive Simulations of Large Molecular and Condensed Systems. Angew. Chem. Int. Ed. 2017, 56, 12828–12840. [Google Scholar] [CrossRef] [PubMed]
- Friederich, P.; Häse, F.; Proppe, J.; Aspuru-Guzik, A. Machine-learned potentials for next-generation matter simulations. Nat. Mater. 2021, 20, 750–761. [Google Scholar] [CrossRef]
- Deringer, V.L.; Caro, M.A.; Csányi, G. Machine Learning Interatomic Potentials as Emerging Tools for Materials Science. Adv. Mater. 2019, 31, 1902765. [Google Scholar] [CrossRef] [PubMed]
- Ronneberger, I.; Zhang, W.; Eshet, H.; Mazzarello, R. Crystallization Properties of the Ge2Sb2Te5 Phase-Change Compound from Advanced Simulations. Adv. Funct. Mater. 2015, 25, 6407–6413. [Google Scholar] [CrossRef]
- Laio, A.; Parrinello, M. Escaping free-energy minima. Proc. Natl. Acad. Sci. USA 2002, 99, 12562–12566. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou El Kheir, O.; Bernasconi, M. High-Throughput Calculations on the Decomposition Reactions of Off-Stoichiometry GeSbTe Alloys for Embedded Memories. Nanomaterials 2021, 11, 2382. https://doi.org/10.3390/nano11092382
Abou El Kheir O, Bernasconi M. High-Throughput Calculations on the Decomposition Reactions of Off-Stoichiometry GeSbTe Alloys for Embedded Memories. Nanomaterials. 2021; 11(9):2382. https://doi.org/10.3390/nano11092382
Chicago/Turabian StyleAbou El Kheir, Omar, and Marco Bernasconi. 2021. "High-Throughput Calculations on the Decomposition Reactions of Off-Stoichiometry GeSbTe Alloys for Embedded Memories" Nanomaterials 11, no. 9: 2382. https://doi.org/10.3390/nano11092382