Oxidative Desulfurization Catalyzed by Phosphotungstic Acid Supported on Hierarchical Porous Carbons
Abstract
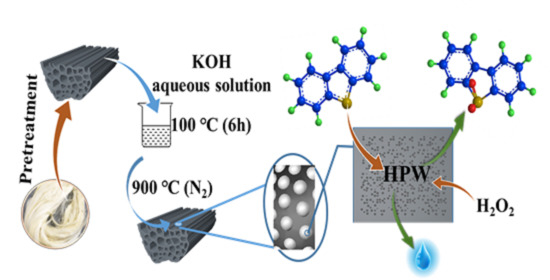
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of HPW/SF-HPC
2.3. Characterization
2.4. Catalytic Performance Testing
3. Results and Discussion
3.1. Catalyst Characterization
3.2. Catalytic Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ogunlaja, A.S.; Coombes, M.J.; Torto, N.; Tshentu, Z.R. The adsorptive extraction of oxidized sulfur-containing compounds from fuels by using molecularly imprinted chitosan materials. React. Funct. Polym. 2014, 81, 61–76. [Google Scholar] [CrossRef]
- Yu, F.L.; Wang, Y.Y.; Liu, C.Y.; Xie, C.X.; Yu, S.T. Oxidative desulfurization of fuels catalyzed by ammonium oxidative-thermoregulated bifunctional ionic liquids. Chem. Eng. J. 2014, 255, 372–376. [Google Scholar] [CrossRef]
- Sachdeva, T.O.; Pant, K.K. Deep desulfurization of diesel via peroxide oxidation using phosphotungstic acid as phase transfer catalyst. Fuel Process. Technol. 2010, 91, 1133–1138. [Google Scholar] [CrossRef]
- Choi, A.E.S.; Roces, S.; Dugos, N.; Wan, M.W. Mixing-assisted oxidative desulfurization of model sulfur compounds using polyoxometalate/H2O2 catalytic system. Sustain. Environ. Res. 2016, 26, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Wu, N.J.; Li, B.S.; Ma, W.; Han, C.Y. Synthesis of lacunary polyoxometalate encapsulated into hexagonal mesoporous silica and their catalytic performance in esterification. Microporous Mesoporous Mater. 2014, 186, 155–162. [Google Scholar] [CrossRef]
- Yang, P.; Zhou, S.Y.; Du, Y.; Li, J.S.; Lei, J.H. Synthesis of ordered meso/macroporous H3PW12O40/SiO2 and its catalytic performance in oxidative desulfurization. RSC Adv. 2016, 6, 53860–53866. [Google Scholar] [CrossRef]
- Du, Y.; Lei, J.H.; Yang, P.; Li, J.S.; Du, X.D. Hierarchical ordered meso/macroporous H3PW12O40/SiO2, catalysts with superior oxidative desulfurization activity. J. Porous Mater. 2018, 25, 727–734. [Google Scholar] [CrossRef]
- Huang, P.C.; Liu, A.L.; Kang, L.H.; Zhu, M.Y.; Dai, B. Heteropoly acid supported on sodium dodecyl benzene sulfonate modified layered double hydroxides as catalyst for oxidative desulfurization. New J. Chem. 2018, 42, 12830–12837. [Google Scholar] [CrossRef]
- Pham, X.N.; Doan, H.V. Activity and stability of amino-functionalized SBA-15 immobilized 12-tungstophosphoric acid in the oxidative desulfurization of a diesel fuel model with H2O2. Chem. Eng. Commun. 2018, 9, 1139–1151. [Google Scholar] [CrossRef]
- Gao, H.C.; Wu, X.N.; Sun, D.M.; Niu, G.L.; Guan, J.Y.; Meng, X.F.; Liu, C.Z.; Xia, W.D.; Song, X.J. Preparation of Core-shell PW12@TiO2 Microsphere and Oxidative Desulfurization Performance. Dalton Trans. 2019, 48, 5749–5755. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Zhu, M.Y.; Kang, L.H.; Yu, F.; Dai, B. Phosphotungstic Acid Supported on Mesoporous Graphitic Carbon Nitride as Catalyst for Oxidative Desulfurization of Fuel. Ind. Eng. Chem. Res. 2015, 54, 2040–2047. [Google Scholar] [CrossRef]
- Du, Y.; Lei, J.H.; Zhou, L.N.; Du, X.D.; Guo, Z.R.; Li, J.S. Oxidative desulfurization of fuels at room temperature using ordered meso/macroporous H3PW12O40/SiO2 catalyst with high specific surface areas. Arab. J. Chem. 2020, 13, 2649–2658. [Google Scholar] [CrossRef]
- Li, S.W.; Gao, R.M.; Zhang, W.; Zhang, Y.; Zhao, J.S. Heteropolyacids supported on macroporous materials POM@MOF-199@LZSM-5: Highly catalytic performance in oxidative desulfurization of fuel oil with oxygen. Fuel 2018, 221, 1–11. [Google Scholar] [CrossRef]
- Huang, P.C.; Luo, G.Q.; Kang, L.H.; Zhu, M.Y.; Dai, B. Preparation, characterization and catalytic performance of HPW/aEVM catalyst on oxidative desulfurization. RSC Adv. 2017, 7, 4681–4687. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.R.; Wu, B.M.; Wu, D.C.; Xu, F.; Li, Z.H.; Luo, J.W.; Zhong, H.; Fu, R.W.; Matyjaszewski, K. Ultrahigh surface area hierarchical porous carbons based on natural well-defined macropores in sisal fibers. J. Mater. Chem. 2011, 21, 14424–14427. [Google Scholar] [CrossRef]
- Liu, X.W.; Liu, X.H.; Sun, B.F.; Zhou, H.L.; Fu, A.P.; Wang, Y.Q.; Guo, Y.G.; Guo, P.Z.; Li, H.L. Carbon materials with hierarchical porosity: Effect of template removal strategy and study on their electrochemical properties. Carbon 2018, 130, 680–691. [Google Scholar] [CrossRef]
- Zhu, Z.G.; Ma, J.X.; Ji, C.H.; Liu, Y.; Wang, W.; Cui, F.Y. Nitrogen doped hierarchically structured porous carbon fibers with an ultrahigh specific surface area for removal of organic dyes. RSC Adv. 2018, 8, 19116–19124. [Google Scholar] [CrossRef] [Green Version]
- Amosa, M.K.; Jami, M.S.; Alkhatib, M.F.R. Electrostatic Biosorption of COD, Mn and H2S on EFB-Based Activated Carbon Produced through Steam Pyrolysis: An Analysis Based on Surface Chemistry. Equilibria and Kinetics. Waste Biomass Valorization 2016, 7, 109–124. [Google Scholar] [CrossRef]
- Choi, A.E.S.; Roces, S.; Dugos, N.; Arcega, A.; Wan, M.W. Adsorptive removal of dibenzothiophene sulfone from fuel oil using clay material adsorbents. J. Clean. Prod. 2017, 161, 267–276. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Bagheri, M.; Morsali, A. Application of Two Cobalt-Based Metal-Organic Frameworks as Oxidative Desulfurization Catalysts. Inorg. Chem. 2015, 54, 11269–11275. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G.; Kong, L.H.; Lu, H. Deep oxidative desulfurization catalyzed by Ti-based metal-organic frameworks. Fuel 2018, 219, 103–110. [Google Scholar] [CrossRef]
- Rose, M.; Korenblit, Y.; Kockrick, E.; Borchardt, L.; Oschatz, M.; Kaskel, S.; Yushin, G. Hierarchical Micro-and Mesoporous Carbide-Derived Carbon as a High-Performance Electrode Material in Supercapacitors. Small 2011, 7, 1108–1117. [Google Scholar] [CrossRef]
- Liang, Y.R.; Wu, D.C.; Fu, R.W. Preparation and electrochemical performance of novel ordered mesoporous carbon with an interconnected channel structure. Langmuir 2009, 25, 7783–7785. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.C.; Liang, Y.R.; Yang, X.Q.; Zou, C.; Li, Z.H.; Lv, G.F.; Zeng, X.H.; Fu, R.W. Preparation of activated ordered mesoporous carbons with a channel structure. Langmuir 2008, 24, 2967–2969. [Google Scholar] [CrossRef]
- Li, B.S.; Liu, Z.X.; Han, C.Y.; Ma, W.; Zhao, S.J. In situ synthesis, characterization, and catalytic performance of tungstophosphoric acid encapsulated into the framework of mesoporous silica pillared clay. J. Colloid. Interf. Sci. 2012, 377, 334–341. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, G.J.; Long, Z.Y.; Wang, J. Recent advances in polyoxometalate-based heterogeneous catalytic materials for liquid-phase organic transformations. RSC Adv. 2014, 4, 42092–42113. [Google Scholar] [CrossRef]
- Wee, L.H.; Bonino, F.; Lambert, C.; Bordiga, S.; Martens, J.A. Cr-MIL-101encapsulated Keggin phosphotungstic acid as active nanomaterial for catalysing the alcoholysis of styrene oxide. Green Chem. 2014, 16, 1351–1357. [Google Scholar] [CrossRef]
- Luo, G.Q.; Kang, L.H.; Zhu, M.Y.; Dai, B. Highly active phosphotungstic acid immobilized on amino functionalized MCM-41 for the oxidesulfurization of dibenzothiophene. Fuel Process. Technol. 2014, 118, 20–27. [Google Scholar] [CrossRef]
- Huang, P.C.; Liu, A.L.; Kang, L.H.; Dai, B.; Zhu, M.Y.; Zhang, J.L. Heteropolyacid Supported on Nitrogen-doped Onion-Like Carbon as Catalyst for Oxidative Desulfurization. ChemistrySelect 2017, 2, 4010–4015. [Google Scholar] [CrossRef]
- Du, Y.; Yang, P.; Zhou, S.Y.; Li, J.S.; Du, X.D.; Lei, J.H. Direct synthesis of ordered meso/macrostructured phosphotungstic acid/SiO2 by EISA method and its catalytic performance of fuel oil. Mater. Res. Bull. 2018, 97, 42–48. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Wang, R. Synthesis of Silica@C-dots/Phosphotungstates Core-Shell Microsphere for Effective Oxidative-Adsorptive Desulfurization of Dibenzothiophene with Less Oxidant. Appl. Catal. B Environ. 2018, 234, 247–259. [Google Scholar] [CrossRef]
- Yan, X.M.; Mei, Z.K.; Mei, P.; Yang, Q.F. Self-assembled HPW/silica–alumina mesoporous nanocomposite as catalysts for oxidative desulfurization of fuel oil. J. Porous Mater. 2014, 21, 729–737. [Google Scholar] [CrossRef]
- Luna, M.D.G.D.; Wan, M.W.; Golosinda, L.; Futalan, C.; Lu, M.C. Kinetics of Mixing-Assisted Oxidative Desulfurization of Dibenzothiophene in Toluene Using a Phosphotungstic Acid/Hydrogen Peroxide System: Effects of Operating Conditions. Energ. Fuel 2017, 31, 9923–9929. [Google Scholar] [CrossRef]
- Han, W.; Rui, W.; Zhang, Y.Q.; Dou, S.Y.; Olga, S.; Vladimir, K. Oxidative Removal of Organo-sulfur Species via H2O2 Oxidation Catalyzed by Lacunary Keggin 11-tungstophosphates. Catal. Lett. 2018, 148, 2501–2509. [Google Scholar] [CrossRef]








| Sample | SBET (m2g−1) | VP (cm3g−1) | d (nm) |
|---|---|---|---|
| SF-HPC | 3152.46 | 1.86 | 2.35 |
| 1%HPW/SF-HPC | 2805.95 | 1.77 | 2.52 |
| 5%HPW/SF-HPC | 2625.28 | 1.57 | 2.40 |
| 10%HPW/SF-HPC | 2436.84 | 1.51 | 2.49 |
| 20%HPW/SF-HPC | 2080.98 | 1.27 | 2.44 |
| Run Number | W (wt %) | HPW (wt %) |
|---|---|---|
| Fresh | 3.257 | 4.252 |
| 1 cycle | 3.129 | 4.085 |
| 2 cycles | 2.878 | 3.757 |
| 3 cycles | 2.616 | 3.415 |
| 4 cycles | 2.198 | 2.870 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Kang, L.; Zhu, M. Oxidative Desulfurization Catalyzed by Phosphotungstic Acid Supported on Hierarchical Porous Carbons. Nanomaterials 2021, 11, 2369. https://doi.org/10.3390/nano11092369
Wang B, Kang L, Zhu M. Oxidative Desulfurization Catalyzed by Phosphotungstic Acid Supported on Hierarchical Porous Carbons. Nanomaterials. 2021; 11(9):2369. https://doi.org/10.3390/nano11092369
Chicago/Turabian StyleWang, Bao, Lihua Kang, and Mingyuan Zhu. 2021. "Oxidative Desulfurization Catalyzed by Phosphotungstic Acid Supported on Hierarchical Porous Carbons" Nanomaterials 11, no. 9: 2369. https://doi.org/10.3390/nano11092369