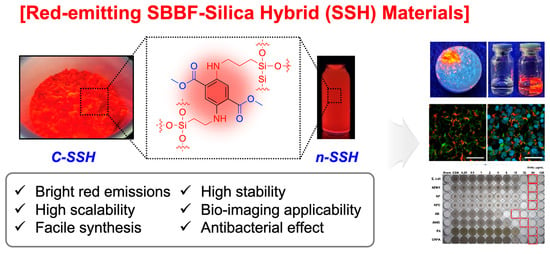
Red-Emitting SBBF (Single-Benzene-Based Fluorophore)-Silica Hybrid Material: One-Pot Synthesis, Characterization, and Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of c-SSH
2.3. Characterization of c-SSH
2.4. Preparation of n-SSH
2.5. Characterization of n-SSH
2.6. Preparation of DCD-propyl
2.7. UV/vis Absorption and Fluorescence Spectroscopic Methods
2.8. Cell Experiment
2.8.1. Cell Culture
2.8.2. Cytotoxicity Analysis
2.8.3. CLSM Cellular Imaging
2.9. Bacterial Experiment
2.9.1. Bacterial Strains and Culture
2.9.2. Minimum Inhibitory Concentration (MIC) Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Rational for the Material Design
3.2. Material Characterization and Photophysical Properties of c-SSH
3.3. Physicochemical Properties and Applications of c-SSH
3.4. Material Characterization and Photophysical Properties of n-SSH
3.5. Biological Applications of n-SSH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Z.; Zhang, H.; Lam, J.W.; Tang, B.Z. Aggregation-induced emission: New vistas at the aggregate level. Angew. Chem. Int. Ed. 2020, 59, 9888–9907. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sharma, A.; Singh, H.; Suating, P.; Kim, H.S.; Sunwoo, K.; Shim, I.; Gibb, B.C.; Kim, J.S. Revisiting fluorescent calixarenes: From molecular sensors to smart materials. Chem. Rev. 2019, 119, 9657–9721. [Google Scholar] [CrossRef]
- Nguyen, V.-N.; Ha, J.; Cho, M.; Li, H.; Swamy, K.; Yoon, J. Recent developments of BODIPY-based colorimetric and fluorescent probes for the detection of reactive oxygen/nitrogen species and cancer diagnosis. Coord. Chem. Rev. 2021, 439, 213936. [Google Scholar] [CrossRef]
- Christoffers, J. Diaminoterephthalate fluorescence dyes–versatile tools for life sciences and materials science. Eur. J. Org. Chem. 2018, 2018, 2366–2377. [Google Scholar] [CrossRef]
- Hong, Y.; Meng, L.; Chen, S.; Leung, C.W.T.; Da, L.-T.; Faisal, M.; Silva, D.-A.; Liu, J.; Lam, J.W.Y.; Huang, X. Monitoring and inhibition of insulin fibrillation by a small organic fluorogen with aggregation-induced emission characteristics. J. Am. Chem. Soc. 2012, 134, 1680–1689. [Google Scholar] [CrossRef]
- Verwilst, P.; Kim, H.S.; Kim, S.; Kang, C.; Kim, J.S. Shedding light on tau protein aggregation: The progress in developing highly selective fluorophores. Chem. Soc. Rev. 2018, 47, 2249–2265. [Google Scholar] [CrossRef]
- Grzybowski, M.; Gryko, D.T. Diketopyrrolopyrroles: Synthesis, reactivity, and optical properties. Adv. Opt. Mater. 2015, 3, 280–320. [Google Scholar] [CrossRef]
- Feng, G.; Kwok, R.T.; Tang, B.Z.; Liu, B. Functionality and versatility of aggregation-induced emission luminogens. Appl. Phys. Rev. 2017, 4, 021307. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, W.; Chung, J.; Yin, J.; Yoon, J. Recent progress in fluorescent probes for bacteria. Chem. Soc. Rev. 2021, 50, 7725–7744. [Google Scholar] [CrossRef]
- Li, W.; Yao, W.; Tebyetekerwa, M.; Tang, J.; Yang, S.; Zhu, M.; Hu, R.; Qin, A.; Tang, B.Z.; Xu, Z. An attempt to adopt aggregation-induced emission to study organic–inorganic composite materials. J. Mater. Chem. C 2018, 6, 7003–7011. [Google Scholar] [CrossRef]
- Hoffmann, H.S.; Stefani, V.; Benvenutti, E.V.; Costa, T.M.H.; Gallas, M.R. Fluorescent silica hybrid materials containing benzimidazole dyes obtained by sol–gel method and high pressure processing. Mater. Chem. Phys. 2011, 126, 97–101. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, R.; Cui, Q.; Xu, W.; Peng, R.; Wang, J.; Li, L. Red-emissive conjugated oligomer/silica hybrid nanoparticles with high affinity and application for latent fingerprint detection. Colloids Surf. A Physicochem. Eng. Asp. 2019, 565, 118–130. [Google Scholar] [CrossRef]
- Shin, J.; Verwilst, P.; Choi, H.; Kang, S.; Han, J.; Kim, N.H.; Choi, J.G.; Oh, M.S.; Hwang, J.S.; Kim, D. Harnessing intramolecular rotation to enhance two-photon imaging of Aβ plaques through minimizing background fluorescence. Angew. Chem. Int. Ed. 2019, 131, 5704–5708. [Google Scholar] [CrossRef]
- Jung, Y.; Ju, I.G.; Choe, Y.H.; Kim, Y.; Park, S.; Hyun, Y.-M.; Oh, M.S.; Kim, D. Hydrazine exposé: The next-generation fluorescent probe. ACS Sens. 2019, 4, 441–449. [Google Scholar] [CrossRef]
- An, J.M.; Moon, H.; Verwilst, P.; Shin, J.; Kim, B.M.; Park, C.-K.; Kim, J.S.; Yeo, S.G.; Kim, H.Y.; Kim, D. Human Glioblastoma Visualization: Triple Receptor-Targeting Fluorescent Complex of Dye, SIWV Tetra-Peptide, and Serum Albumin Protein. ACS Sens. 2021, 6, 2270–2280. [Google Scholar] [CrossRef]
- Kang, S.; Jung, Y.; Jung, J.; Park, K.-H.; Kim, D. Bacteria-dye combination screening: Diamine-containing BMeS-pA dye for specific fluorescence imaging of Acinetobacter baumannii. Dyes Pigm. 2021, 185, 108939. [Google Scholar] [CrossRef]
- Kim, J.; Oh, J.H.; Kim, D. Recent advances in single-benzene-based fluorophores: Physicochemical properties and applications. Org. Biomol. Chem. 2021, 19, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Wache, N.; Schröder, C.; Koch, K.W.; Christoffers, J. Diaminoterephthalate Turn-On Fluorescence Probes for Thiols—Tagging of Recoverin and Tracking of its Conformational Change. ChemBioChem 2012, 13, 993–998. [Google Scholar] [CrossRef]
- Shindy, H. Fundamentals in the chemistry of cyanine dyes: A review. Dyes Pigm. 2017, 145, 505–513. [Google Scholar] [CrossRef]
- Shimizu, M.; Takeda, Y.; Higashi, M.; Hiyama, T. 1, 4-Bis (alkenyl)-2, 5-dipiperidinobenzenes: Minimal Fluorophores Exhibiting Highly Efficient Emission in the Solid State. Angew. Chem. Int. Ed. 2009, 48, 3653–3656. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, H.; Fan, Y.; Zhou, T.; Su, Z.; Liu, Y.; Wang, Y. Thermally induced reversible phase transformations accompanied by emission switching between different colors of two aromatic-amine compounds. Adv. Mater. 2009, 21, 3165–3169. [Google Scholar] [CrossRef]
- Tang, B.; Wang, C.; Wang, Y.; Zhang, H. Efficient Red-Emissive Organic Crystals with Amplified Spontaneous Emissions Based on a Single Benzene Framework. Angew. Chem. Int. Ed. 2017, 129, 12717–12721. [Google Scholar] [CrossRef]
- Zhou, R.; Cui, Y.; Dai, J.; Wang, C.; Liang, X.; Yan, X.; Liu, F.; Liu, X.; Sun, P.; Zhang, H. A Red-Emissive Fluorescent Probe with a Compact Single-Benzene-Based Skeleton for Cell Imaging of Lipid Droplets. Adv. Opt. Mater. 2020, 8, 1902123. [Google Scholar] [CrossRef]
- Shimizu, M.; Asai, Y.; Takeda, Y.; Yamatani, A.; Hiyama, T. Twisting strategy applied to N, N-diorganoquinacridones leads to organic chromophores exhibiting efficient solid-state fluorescence. Tetrahedron Lett. 2011, 52, 4084–4089. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Wang, C.; Wang, Y.; Zhang, H. Elastic Self-Doping Organic Single Crystals Exhibiting Flexible Optical Waveguide and Amplified Spontaneous Emission. Adv. Mater. 2018, 30, 1800814. [Google Scholar] [CrossRef] [PubMed]
- Maity, P.; Kasisomayajula, S.V.; Parameswaran, V.; Basu, S.; Gupta, N. Improvement in surface degradation properties of polymer composites due to pre-processed nanometric alumina fillers. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 63–72. [Google Scholar] [CrossRef]
- Munajad, A.; Subroto, C. Fourier transform infrared (FTIR) spectroscopy analysis of transformer paper in mineral oil-paper composite insulation under accelerated thermal aging. Energies 2018, 11, 364. [Google Scholar] [CrossRef] [Green Version]
- Pletsch, D.; da Silveira Santos, F.; Rodembusch, F.S.; Stefani, V.; Campo, L.F. Bis-silylated terephthalate as a building block precursor for highly fluorescent organic–inorganic hybrid materials. New. J. Chem. 2012, 36, 2506–2513. [Google Scholar] [CrossRef]
- Wang, T.-H.; Gole, J.L.; White, M.G.; Watkins, C.; Street, S.C.; Fang, Z.; Dixon, D.A. The surprising oxidation state of fumed silica and the nature of water binding to silicon oxides and hydroxides. Chem. Phys. Lett. 2011, 501, 159–165. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret FTIR spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Wang, H.; Lu, F.; Ma, C.; Ma, Y.; Zhang, M.; Wang, B.; Zhang, Y.; Liu, Y.; Huang, H.; Kang, Z. Carbon dots with positive surface charge from tartaric acid and m-aminophenol for selective killing of Gram-positive bacteria. J. Mater. Chem. B 2021, 9, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, N.; Hawash, M.; Abualhasan, M.N.; Qadi, M.; Ghanim, M.; Massarwy, E.; Ammar, S.A.; Zmero, N.; Arar, M.; Hussein, F.; et al. Spectral characterization, antioxidant, antimicrobial, cytotoxic, and cyclooxygenase inhibitory activities of Aloysia citriodora essential oils collected from two Palestinian regions. BMC Complement Altern. Med. 2021, 21, 143. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; An, J.M.; Jung, Y.; Kim, N.H.; Kim, Y.; Kim, D. Red-Emitting SBBF (Single-Benzene-Based Fluorophore)-Silica Hybrid Material: One-Pot Synthesis, Characterization, and Biomedical Applications. Nanomaterials 2021, 11, 2036. https://doi.org/10.3390/nano11082036
Kim J, An JM, Jung Y, Kim NH, Kim Y, Kim D. Red-Emitting SBBF (Single-Benzene-Based Fluorophore)-Silica Hybrid Material: One-Pot Synthesis, Characterization, and Biomedical Applications. Nanomaterials. 2021; 11(8):2036. https://doi.org/10.3390/nano11082036
Chicago/Turabian StyleKim, Jaehoon, Jong Min An, Yuna Jung, Na Hee Kim, Youngwoong Kim, and Dokyoung Kim. 2021. "Red-Emitting SBBF (Single-Benzene-Based Fluorophore)-Silica Hybrid Material: One-Pot Synthesis, Characterization, and Biomedical Applications" Nanomaterials 11, no. 8: 2036. https://doi.org/10.3390/nano11082036