Ionic Liquids for Development of Heterogeneous Catalysts Based on Nanomaterials for Biocatalysis
Abstract
:1. Introduction
2. Stabilization of Enzymes via Ionic Liquids
3. Supported Ionic Liquid Phases in Biocatalysis
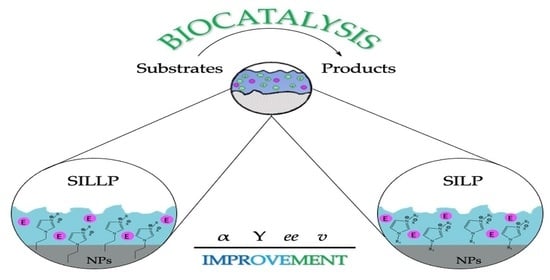
4. Contribution of Nanomaterials to Effectiveness of Supported Ionic Liquid Phases
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Scheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Gottardo, S.; Mech, A.; Riego, S.; Sintes, J.; Rauscher, H.; Drbohlavova, J.; Malyska, A.; Bowadt, S. Towards safe and sustainable in-novation in nanotechnology: State-of-play for smart nanomaterials. NanoImpact 2021, 21, 100297. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Arends, I.; Hanefeld, U. Green Chemistry and Catalysis; Wiley-VCH Verlag GmbH: Berlin, Germany, 2020. [Google Scholar]
- Scheldon, R.A.; Woodley, J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Enzyme immobilization and its applications. Molecules 2019, 24, 4619. [Google Scholar] [CrossRef] [Green Version]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotec. Eq. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Moehlenbrock, M.J.; Minteer, S.D. Introduction to the field of enzyme immobilization and stabilization. Methods Mol. Biol. 2016, 1–7. [Google Scholar] [CrossRef]
- Weiser, D.; Sóti, P.L.; Bánóczi, G.; Bódai, V.; Kiss, B.; Gellért, Á.; Nagy, Z.K.; Koczka, B.; Szilagyi, A.; Marosi, G.; et al. Bioimprinted lipases in PVA nanofibers as efficient immobilized biocatalysts. Tetrahedron 2016, 72, 7335–7342. [Google Scholar] [CrossRef] [Green Version]
- Fotiadou, R.; Patila, M.; Hammami, M.A.; Enotiadis, A.; Moschovas, D.; Tsirka, K.; Spyrou, K.; Giannelis, E.P.; Avgeropoulos, A.; Paipetis, A.; et al. Development of effective lipase-hybrid nanoflowers enriched with carbon and magnetic nanomaterials for biocatalytic transformations. Nanomaterials 2019, 9, 808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabhavathi Devi, B.L.A.; Guo, Z.; Xu, X. Characterization of cross-linked lipase aggregates. J. Am. Oil Chem. Soc. 2009, 86, 637–642. [Google Scholar] [CrossRef]
- Liu, P.; Liu, X.; An, N.; Wang, P. Synthesis of mesoporous silica nanowires and their application in enzyme immobilization. E3S Web Conf. 2021, 245. [Google Scholar] [CrossRef]
- Drożdż, A.; Chrobok, A.; Baj, S.; Szymańska, K.; Mrowiec-Białoń, J.; Jarzębski, A.B. The chemo-enzymatic Baeyer–Villiger oxidation of cyclic ketones with an efficient silica-supported lipase as a biocatalyst. Appl. Catal. A Gen. 2013, 467, 163–170. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, C.; Gao, H.; Xia, H.; Mahmood, I.; Liu, C.; Liu, H. Lipase immobilization on ionic liquid-modified magnetic nanoparticles: Ionic liquids controlled esters hydrolysis at oil-water interface. AIChE J. 2011, 58, 1203–1211. [Google Scholar] [CrossRef]
- Suo, H.; Xu, L.; Xu, C.; Qiu, X.; Huang, H.; Hu, Y. Enhanced catalytic performance of lipase covalently bonded on ionic liquids modified magnetic alginate composites. J. Colloid Interf. Sci. 2019, 553, 494–502. [Google Scholar] [CrossRef]
- Suo, H.; Xu, L.; Xue, Y.; Qiu, X.; Huang, H.; Hu, Y. Ionic liquids-modified cellulose coated magnetic nanoparticles for enzyme immobilization: Improvement of catalytic performance. Carbohydr. Polym. 2020, 234, 115914. [Google Scholar] [CrossRef]
- Carvalho, N.; Vidal, B.; Barbosa, A.; Pereira, M.; Mattedi, S.; Freitas, L.; Lima, A.S.; Soares, C. Lipase immobilization on silica xerogel treated with protic ionic liquid and its application in biodiesel production from different oils. Int. J. Mol. Sci. 2018, 19, 1829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutschler, J.; Rausis, T.; Bourgeois, J.-M.; Bastian, C.; Zufferey, D.; Mohrenz, I.V.; Fischer, F. Ionic liquid-coated immobilized lipase for the synthesis of methylglucose fatty acid esters. Green Chem. 2009, 11, 1793. [Google Scholar] [CrossRef]
- He, Y.; Li, J.-J.; Luo, Y.-K.; Song, F.; Wang, X.-L.; Wang, Y.-Z. Coating Novozyme435 with an ionic liquid: More than just a coating for the efficient ring-opening polymerization of δ-valerolactone. RSC Adv. 2015, 5, 68276–68282. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Z.; Chen, C.; He, F.; Zhuo, R. Synthesis of poly(ε-caprolactone) by an immobilized lipase coated with ionic liquids in a solvent-free condition. Biotechnol. Lett. 2013, 35, 1623–1630. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, S.; Miao, S.; Suo, H.; Xu, H.; Hu, Y. Co-immobilization of Laccase and ABTS onto amino-functionalized ionic liquid-modified magnetic chitosan nanoparticles for pollutants removal. J. Hazard. Mater. 2020, 123353. [Google Scholar] [CrossRef]
- Liu, X.; Bu, C.; Nan, Z.; Zheng, L.; Qiu, Y.; Lu, X. Enzymes immobilized on amine-terminated ionic liquid-functionalized carbon nanotube for hydrogen peroxide determination. Talanta 2013, 105, 63–68. [Google Scholar] [CrossRef]
- Brondani, D.; Zapp, E.; Vieira, I.C.; Dupont, J.; Scheeren, C.W. Gold nanoparticles in an ionic liquid phase supported in a biopolymeric matrix applied in the development of a rosmarinic acid biosensor. Analyst 2011, 136, 2495. [Google Scholar] [CrossRef]
- Abdelrahim, M.Y.M.; Martins, C.F.; Neves, L.A.; Capasso, C.; Supuran, C.T.; Coelhoso, I.M.; Crespo, J.G.; Barboiu, M. Supported ionic liquid membranes immobilized with carbonic anhydrases for CO2 transport at high temperatures. J. Membr. Sci. 2017, 528, 225–230. [Google Scholar] [CrossRef]
- Lozano, P.; Diego, T.; de Carrié, D.; Vaultier, M.; Iborra, J.L. Continuous green biocatalytic processes using ionic liquids and supercritical carbon dioxide. Chem. Commun. 2002, 7, 692–693. [Google Scholar] [CrossRef] [PubMed]
- Lozano, P.; De Diego, T.; Carrie, D.; Vaultier, M.; Iborra, J.L. Lipase catalysis in ionic liquids and supercritical carbon dioxide at 150 °C. Biotechnol. Prog. 2003, 19, 380–382. [Google Scholar] [CrossRef]
- Sandig, B.; Michalek, L.; Vlahovic, S.; Antonovici, M.; Hauer, B.; Buchmeiser, M.R. A Monolithic hybrid cellulose-2.5-Acetate/polymer bioreactor for biocatalysis under continuous liquid-liquid conditions using a supported ionic liquid phase. Chem. Eur. J. 2015, 21, 15835–15842. [Google Scholar] [CrossRef] [PubMed]
- Lozano, P.; García-Verdugo, E.; Piamtongkam, R.; Karbass, N.; De Diego, T.; Burguete, M.I.; Luis, S.V.; Iborra, J.L. Bioreactors based on monolith-supported ionic liquid phase for enzyme catalysis in supercritical carbon dioxide. Adv. Synth. Catal. 2007, 349, 1077–1084. [Google Scholar] [CrossRef]
- Izquierdo, D.F.; Bernal, J.M.; Burguete, M.I.; García-Verdugo, E.; Lozano, P.; Luis, S.V. An efficient microwave-assisted enzymatic resolution of alcohols using a lipase immobilised on supported ionic liquid-like phases (SILLPs). RSC Adv. 2013, 3, 13123. [Google Scholar] [CrossRef]
- Lozano, P.; García-Verdugo, E.; Bernal, J.M.; Izquierdo, D.F.; Burguete, M.I.; Sánchez-Gómez, G.; Luis, S.V. Immobilised lipase on structured supports containing covalently attached ionic liquids for the continuous synthesis of biodiesel in scCO2. ChemSusChem 2012, 5, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Domínguez de María, P. Ionic Liquids in Biotransformations and Organocatalysis: Solvents and Beyond; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 1–435. [Google Scholar]
- Garcia-Verdugo, E.; Lozano, P.; Luis, S.V. Biocatalytic Processes Based on Supported Ionic Liquids. In Supported Ionic Liquids: Fundamental and Applications, 1st ed.; Fehrmann, R., Riisager, A., Haumann, M., Eds.; Wiley-VCH Verlag GmbH: Berlin, Germany, 2014; pp. 351–368. [Google Scholar]
- García-Verdugo, E.; Altava, B.; Burguete, M.I.; Lozano, P.; Luis, S.V. Ionic liquids and continuous flow processes: A good marriage to design sustainable processes. Green Chem. 2015, 17, 2693–2713. [Google Scholar] [CrossRef] [Green Version]
- Potdar, M.; Kelso, G.; Schwarz, L.; Zhang, C.; Hearn, M. Recent developments in chemical synthesis with biocatalysts in ionic liquids. Molecules 2015, 20, 16788–16816. [Google Scholar] [CrossRef]
- Vekariya, R.L. A review of ionic liquids: Applications towards catalytic organic transformations. J. Mol. Liq. 2017, 227, 44–60. [Google Scholar] [CrossRef]
- Amarasekara, A.S. Acidic Ionic Liquids. Chem. Rev. 2016, 116, 6133–6183. [Google Scholar] [CrossRef]
- Greer, A.J.; Jacquemin, J.; Hardacre, C. Industrial applications of ionic liquids. Molecules 2020, 25, 5207. [Google Scholar] [CrossRef]
- Matuszek, K.; Chrobok, A.; Latos, P.; Markiton, M.; Szymańska, K.; Jarzębski, A.; Swadźba-Kwaśny, M. Silica-supported chlorometallate(iii) ionic liquids as recyclable catalysts for Diels–Alder reaction under solventless conditions. Catal. Sci. Technol. 2016, 6, 8129–8137. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Luo, J.; Sun, T.; Yu, F.; Li, C. The absorption performance of ionic liquids–PEG200 complex absorbent for VOCs. Energies 2021, 14, 3592. [Google Scholar] [CrossRef]
- Bajkacz, S.; Rusin, K.; Wolny, A.; Adamek, J.; Erfurt, K.; Chrobok, A. Highly efficient extraction procedures based on natural deep eutectic solvents or ionic liquids for determination of 20-Hydroxyecdysone in Spinach. Molecules 2020, 25, 4736. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Kamiya, N.; Goto, M. Activation and stabilization of enzymes in ionic liquids. Org. Biomol. Chem. 2010, 8, 2887. [Google Scholar] [CrossRef] [PubMed]
- Szelwicka, A.; Chrobok, A. Methods for increasing activity and stability of enzymes in processes carried out in presence of ionic liquids. Przem. Chem. 2018, 97, 89–93. [Google Scholar] [CrossRef]
- Lee, S.H.; Doan, T.T.N.; Ha, S.H.; Chang, W.-J.; Koo, Y.-M. Influence of ionic liquids as additives on sol–gel immobilized lipase. J. Mol. Catal. B Enzym. 2007, 47, 129–134. [Google Scholar] [CrossRef]
- Shah, S.; Gupta, M.N. Kinetic resolution of (±)-1-phenylethanol in [Bmim][PF6] using high activity preparations of lipases. Bioorg. Med. Chem. Lett. 2007, 17, 921–924. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Kamiya, N.; Nakashima, K.; Goto, M. Water-in-ionic liquid microemulsions as a new medium for enzymatic reactions. Green Chem. 2008, 10, 497. [Google Scholar] [CrossRef]
- Lozano, P.; Piamtongkam, R.; Kohns, K.; Diego, T.D.; Vaultier, M.; Iborra, J.L. Ionic liquids improve citronellyl ester synthesis catalyzed by immobilized Candida antarctica lipase B in solvent-free media. Green Chem. 2007, 9, 780. [Google Scholar] [CrossRef]
- Itoh, T. Ionic liquids as tool to improve enzymatic organic synthesis. Chem. Rev. 2017, 117, 10567–10607. [Google Scholar] [CrossRef]
- Zhao, H. Methods for stabilizing and activating enzymes in ionic liquids—A review. J. Chem. Technol. Biotechnol. 2010, 85, 891–907. [Google Scholar] [CrossRef]
- De Los Ríos, A.P.; Hernández-Fernández, F.J.; Martínez, F.A.; Rubio, M.; Víllora, G. The effect of ionic liquid media on activity, selectivity and stability of Candida antarcticalipase B in transesterification reactions. Biocatal. Biotransform. 2007, 25, 151–156. [Google Scholar] [CrossRef]
- Nara, S.J.; Harjani, J.R.; Salunkhe, M.M. Lipase-catalysed transesterification in ionic liquids and organic solvents: A comparative study. Tetrahedron Lett. 2002, 43, 2979–2982. [Google Scholar] [CrossRef]
- Nakashima, K.; Okada, J.; Maruyama, T.; Kamiya, N.; Goto, M. Activation of lipase in ionic liquids by modification with comb-shaped poly(ethylene glycol). Sci. Technol. Adv. Mater. 2006, 7, 692–698. [Google Scholar] [CrossRef]
- Zhang, W.-G.; Wei, D.-Z.; Yang, X.-P.; Song, Q.-X. Penicillin acylase catalysis in the presence of ionic liquids. Bioprocess Biosyst. Eng. 2006, 29, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kazlauskas, R.J. Improved preparation and use of room-temperature ionic liquids in lipase-catalyzed enantio- and regioselective acylations. J. Org. Chem. 2001, 66, 8395–8401. [Google Scholar] [CrossRef] [PubMed]
- De Diego, T.; Lozano, P.; Abad, M.A.; Steffensky, K.; Vaultier, M.; Iborra, J.L. On the nature of ionic liquids and their effects on lipases that catalyze ester synthesis. J. Biotechnol. 2009, 140, 234–241. [Google Scholar] [CrossRef]
- Turner, M.B.; Spear, S.K.; Huddleston, J.G.; Holbrey, J.D.; Rogers, R.D. Ionic liquid salt-induced inactivation and unfolding of cellulase from Trichoderma reesei. Green Chem. 2003, 5, 443. [Google Scholar] [CrossRef]
- Lee, S.H.; Ha, S.H.; Lee, S.B.; Koo, Y.-M. Adverse effect of chloride impurities on lipase-catalyzed transesterifications in ionic liquids. Biotechnol. Lett. 2006, 28, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Lozano, P.; deDiego, T.; Gmouh, S.; Vaultier, M.; Iborra, J.L. Criteria to design green enzymatic processes in ionic liquid/supercritical carbon dioxide systems. Biotechnol. Prog. 2004, 20, 661–669. [Google Scholar] [CrossRef]
- Lozano, P. Synthesis of glycidyl esters catalyzed by lipases in ionic liquids and supercritical carbon dioxide. J. Mol. Catal. A Chem. 2004, 214, 113–119. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Hernandez, K.; Barbosa, O.; Rueda, N.; Garcia-Galan, C.; dosSantos, J.C.S.; Berenguer-Murcia, A.; Fernandez-Lafuente, R. Immobilization of proteins in poly-styrene-divinylbenzene matrices: Functional properties and applications. Curr. Org. Chem. 2015, 19, 1707–1718. [Google Scholar] [CrossRef] [Green Version]
- Lozano, P.; De Diego, T.; Mira, C.; Montague, K.; Vaultier, M.; Iborra, J.L. Long term continuous chemoenzymatic dynamic kinetic resolution of rac-1-phenylethanol using ionic liquids and supercritical carbon dioxide. Green Chem. 2009, 11, 538. [Google Scholar] [CrossRef]
- Lozano, P.; García-Verdugo, E.; Karbass, N.; Montague, K.; De Diego, T.; Burguete, M.I.; Luis, S.V. Supported ionic liquid-like phases (SILLPs) for enzymatic processes: Continuous KR and DKR in SILLP–scCO2 systems. Green Chem. 2010, 12, 1803. [Google Scholar] [CrossRef]
- Izquierdo, D.F.; Yates, M.; Lozano, P.; Burguete, M.I.; García-Verdugo, E.; Luis, S.V. Macroporous polymers tailored as supports for large biomolecules: Ionic liquids as porogenic solvents and as surface modifiers. React. Funct. Polym. 2014, 85, 20–27. [Google Scholar] [CrossRef]
- Kim, H.; Hassouna, F.; Muzika, F.; Arabacı, M.; Kopecký, D.; Sedlářová, I.; Šoóš, M. Urease adsorption immobilization on ionic liquid-like macroporous polymeric support. J. Mater. Sci. 2019, 54, 14884–14896. [Google Scholar] [CrossRef]
- Porcar, R.; Lavandera, I.; Lozano, P.; Altava, B.; Luis, S.V.; Gotor-Fernández, V.; García-Verdugo, E. Supported ionic liquid-like phases as efficient solid ionic solvent for the immobilisation of alcohol dehydrogenases towards the development of stereoselective bioreductions. Green Chem. 2021. [Google Scholar] [CrossRef]
- Sandig, B.; Buchmeiser, M.R. Highly productive and enantioselective enzyme catalysis under continuous supported liquid-liquid conditions using a hybrid monolithic bioreactor. ChemSusChem 2016, 9, 2917–2921. [Google Scholar] [CrossRef]
- Lee, C.; Sandig, B.; Buchmeiser, M.R.; Haumann, M. Supported ionic liquid phase (SILP) facilitated gas-phase enzyme catalysis–CALB catalyzed transesterification of vinyl propionate. Catal. Sci. Technol. 2018, 8, 2460–2466. [Google Scholar] [CrossRef]
- Jaworska, M.M.; Stepniak, I.; Galiński, M.; Kasprzak, D.; Biniaś, D.; Górak, A. Modification of chitin structure with tailored ionic liquids. Carbohydr. Polym. 2018, 202, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Ding, S.; Suo, H.; Xu, C.; Gao, Z.; Hu, Y. Fabrication of chitosan-mesoporous silica SBA-15 nanocomposites via functional ionic liquid as the bridging agent for PPL immobilization. Carbohydr. Polym. 2018, 182, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Suo, H.; Xu, L.; Xu, C.; Chen, H.; Yu, D.; Gao, Z.; Huang, H.; Hu, Y. Enhancement of catalytic performance of porcine pancreatic lipase immobilized on functional ionic liquid modified Fe3O4-chitosan nanocomposites. Int. J. Biol. Macromol. 2018, 119, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Guo, C.; Xia, H.; Mahmood, I.; Liu, C.; Liu, H. Magnetic nanoparticles supported ionic liquids for lipase immobilization: Enzyme activity in catalyzing esterification. J. Mol. Catal. B Enzym. 2009, 58, 103–109. [Google Scholar] [CrossRef]
- Zhou, H.; Li, W.; Shou, Q.; Gao, H.; Xu, P.; Deng, F.; Liu, H. Immobilization of Penicillin G Acylase on magnetic nanoparticles modified by ionic liquids. Chin. J. Chem. Eng. 2012, 20, 146–151. [Google Scholar] [CrossRef]
- Xie, W.; Zang, X. Lipase immobilized on ionic liquid-functionalized magnetic silica composites as a magnetic biocatalyst for production of trans -free plastic fats. Food Chem. 2018, 257, 15–22. [Google Scholar] [CrossRef]
- Iwanow, M.; Gärtner, T.; Sieber, V.; König, B. Activated carbon as catalyst support: Precursors, preparation, modification and characterization. Beilstein J. Org. Chem. 2020, 16, 1188–1202. [Google Scholar] [CrossRef]
- Hara, P.; Mikkola, J.-P.; Murzin, D.Y.; Kanerva, L.T. Supported ionic liquids in Burkholderia cepacia lipase-catalyzed asymmetric acylation. J. Mol. Catal. B Enzym. 2010, 67, 129–134. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.; Tang, S.; Xiang, X.; Huang, H.; Hu, Y. Immobilization of Candida antarctic Lipase B on functionalized ionic liquid modified MWNTs. Appl. Biochem. Biotechnol. 2017, 183, 807–819. [Google Scholar] [CrossRef]
- Wan, X.; Xiang, X.; Tang, S.; Yu, D.; Huang, H.; Hu, Y. Immobilization of Candida antarctic lipase B on MWNTs modified by ionic liquids with different functional groups. Colloid Surf. B 2017, 160, 416–422. [Google Scholar] [CrossRef]
- Xiang, X.; Wan, X.; Suo, H.; Hu, Y. Study of surface modifications of multiwalled carbon nanotubes by functionalized ionic liquid to immobilize Candida antarctic lipase B. Acta Phys. Chim. Sin. 2018, 34, 99–107. [Google Scholar] [CrossRef]
- Szelwicka, A.; Erfurt, K.; Jurczyk, S.; Boncel, S.; Chrobok, A. Outperformance in Acrylation: Supported D-glucose-based ionic liquid phase on MWCNTs for immobilized lipase B from Candida antarctica as catalytic system. Materials 2021, 14, 3090. [Google Scholar] [CrossRef] [PubMed]
- Szelwicka, A.; Wolny, A.; Grymel, M.; Jurczyk, S.; Boncel, S.; Chrobok, A. Chemo-enzymatic Baeyer–Villiger oxidation facilitated with lipases immobilized in the supported ionic liquid phase. Materials 2021, 14, 3443. [Google Scholar] [CrossRef]
- Costantini, A.; Califano, V. Lipase immobilization in mesoporous silica nanoparticles for biofuel production. Catalysts 2021, 11, 629. [Google Scholar] [CrossRef]
- Zou, B.; Hu, Y.; Yu, D.; Xia, J.; Tang, S.; Liu, W.; Huang, H. Immobilization of porcine pancreatic lipase onto ionic liquid modified mesoporous silica SBA-15. Biochem. Eng. J. 2010, 53, 150–153. [Google Scholar] [CrossRef]
- Zou, B.; Hu, Y.; Yu, D.; Jiang, L.; Liu, W.; Song, P. Functionalized ionic liquid modified mesoporous silica SBA-15: A novel, designable and efficient carrier for porcine pancreas lipase. Colloid Surf. B 2011, 88, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Tang, S.; Jiang, L.; Zou, B.; Yang, J.; Huang, H. Immobilization of Burkholderia cepacia lipase on functionalized ionic liquids modified mesoporous silica SBA-15. Process Biochem. 2012, 47, 2291–2299. [Google Scholar] [CrossRef]
- Yang, J.; Hu, Y.; Jiang, L.; Zou, B.; Jia, R.; Huang, H. Enhancing the catalytic properties of porcine pancreatic lipase by immobilization on SBA-15 modified by functionalized ionic liquid. Biochem. Eng. J. 2013, 70, 46–54. [Google Scholar] [CrossRef]
- Zou, B.; Hu, Y.; Jiang, L.; Jia, R.; Huang, H. Mesoporous material SBA-15 modified by amino acid ionic liquid to immobilize lipase via ionic bonding and cross-linking method. Ind. Eng. Chem. Res. 2013, 52, 2844–2851. [Google Scholar] [CrossRef]
- Zou, B.; Hu, Y.; Cui, F.; Jiang, L.; Yu, D.; Huang, H. Effect of surface modification of low cost mesoporous SiO2 carriers on the properties of immobilized lipase. J. Colloid Interface Sci. 2014, 417, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Song, C.; Xu, X.; Xia, J.; Huo, S.; Cui, F. Enhancing stabilities of lipase by enzyme aggregate coating immobilized onto ionic liquid modified mesoporous materials. Appl. Surf. Sci. 2014, 311, 62–67. [Google Scholar] [CrossRef]
- Zou, B.; Chu, Y.; Xia, J.; Chen, X.; Huo, S. Immobilization of lipase by ionic liquid-modified mesoporous SiO2 adsorption and calcium alginate-embedding method. Appl. Biochem. Biotechnol. 2018, 185, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Bian, W.; Yan, B.; Shi, N.; Qiu, F.; Lou, L.-L.; Qi, B.; Liu, S. Room temperature ionic liquid (RTIL)-decorated mesoporous silica SBA-15 for papain immobilization: RTIL increased the amount and activity of immobilized enzyme. Mater. Sci. Eng. C 2012, 32, 364–368. [Google Scholar] [CrossRef]
- Khademy, M.; Karimi, B.; Zareian, S. Ionic liquid-based periodic mesoporous organosilica: An innovative matrix for enzyme immobilization. Chem. Sel. 2017, 2, 9953–9957. [Google Scholar] [CrossRef] [Green Version]
- Zhong, N.; Li, Y.; Cai, C.; Gao, Y.; Liu, N.; Liu, G.; Tan, W.; Zeng, Y. Enhancing the catalytic performance of Candida antarctica lipase B by immobilization onto the ionic liquids modified SBA-15. Eur. J. Lipid. Sci. Tech. 2018, 120, 1700357. [Google Scholar] [CrossRef]
- Barbosa, A.S.; Lisboa, J.A.; Silva, M.A.O.; Carvalho, N.B.; Pereira, M.M.; Fricks, A.T.; Mattedi, S.; Lima, A.S.; Franceschi, E.; Soares, C.M.F. The novel mesoporous silica aerogel modified with protic ionic liquid for lipase immobilization. Quim. Nova. 2016, 39, 415–422. [Google Scholar] [CrossRef]
- Lisboa, M.C.; Rodrigues, C.A.; Barbosa, A.S.; Mattedi, S.; Freitas, L.S.; Mendes, A.A.; Dariva, C.; Franceschi, E.; Lima, A.S.; Soares, C.M.F. New perspectives on the modification of silica aerogel particles with ionic liquid applied in lipase immobilization with platform in ethyl esters production. Process Biochem. 2018, 75, 157–165. [Google Scholar] [CrossRef]
- Barbosa, M.; Santos, A.; Carvalho, N.B.; Figueiredo, R.; Pereira, M.M.; Lima, Á.S.; Freire, M.G.; Cabrera-Padilla, R.Y.; Soares, C.M.F. Enhanced activity of immobilized lipase by phosphonium-based ionic liquids used in the supports preparation and immobilization process. ACS Sustain. Chem. Eng. 2019, 7, 15648–15659. [Google Scholar] [CrossRef]
- Philippot, K.; Serp, P. Concepts in Nanocatalysis. In Nanomaterials in Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Berlin, Germany, 2012; pp. 1–54. [Google Scholar] [CrossRef]
- Gupta, M.N.; Kaloti, M.; Kapoor, M.; Solanki, K. Nanomaterials as matrices for enzyme immobilization. Artif. Cells Blood Substit. Immobil. Biotechnol. 2010, 39, 98–109. [Google Scholar] [CrossRef]






| Type of Technique | Ionic Liquid | Enzyme | Reaction | Ref. |
|---|---|---|---|---|
| SILC | Imidazolium cation [Cl]− anion | CRL (16 U/mg) 1 | Esters hydrolysis | [13] |
| SILC | Imidazolium cation [PF6]− anion | PPL (659.4 U/g) | Triacetin hydrolysis | [14] |
| SILC | Imidazolium cation [PF6]− anion | PPL (882.1 U/g) | Triacetin hydrolysis | [15] |
| SILC | Ammonium cation [C4H9COO]− anion | BCL (3801 U/g) | Hydrolysis and transesterification of different oils α between 70 and 98% 2 | [16] |
| SCIL | Pyridinium cation [PF6]− anion | CALB 3 | Esterification of fatty acids α between 25 and 65% | [17] |
| SCIL | Imidazolium cation [PF6]− anion | CALB 3 | Ring-opening polymerization of lactone α = 60% | [18] |
| SCIL | Imidazolium cation [NTf2]− anion | CALB 3 | Ring-opening polymerization of lactone Y = 62% 4 | [19] |
| SILP | Imidazolium cation [NTf2]− anion | CALB (1.7 U/mg) | Kinetic resolution of 1-phenylethanol via transesterification ee > 99.9% 5 | [24] |
| SILP | Imidazolium cation [NTf2]− anion | CALB (71 U/mg) | Transesterification of vinyl butyrate S = 99% 6 | [24] |
| SILP | Imidazolium cation [NTf2]− anion | CALB (9.1 U/mg) | Kinetic resolution of 1-phenylethanol via transesterification ee > 99.9% | [25] |
| SILP | Imidazolium cation [BF4]− anion | CALB (58 U/mg) | Transesterification of vinyl butyrate α = 96% | [26] |
| SILLP | Imidazolium cation [Cl]− anion | CALB (20 U/mg) | Transesterification of vinyl propionate Y = 93% | [27] |
| SILLP | Imidazolium cation [NTf2]− anion | CALB (1138.3 U/g) | Kinetic resolution of 1-phenylethanol via transesterification Y = 81%, ee = 94% | [28] |
| SILLP | Imidazolium cation [NTf2]− anion | CALB (49.8 U/g) | Triolein transesterification Y = 85% | [29] |
| Type | Nanomaterial | Ionic Liquid | Enzyme | Reaction | Ref. |
|---|---|---|---|---|---|
| SILLP | Chitosan–silica hybrid | Imidazolium [BF4]− | PLL (2482 U/g 1, 132.1 mg/g 2) | Triacetin hydrolysis 35 °C, 10 cycles | [67] |
| SILLP | Chitosan–Fe3O4 hybrid | Imidazolium [PF6]− | PPL (2879 U/g, 118 mg/g) | Triacetin hydrolysis 50 °C, 10 cycles | [68] |
| SILLP | Fe3O4 | Imidazolium [PF6]− | CRL (132.3 U/g, 639 mg/g) | Oleic acid esterification 30 °C, 5 cycles | [69] |
| SILLP | Fe3O4 | Imidazolium [Cl]− | Penicillin G acylase (261 U/g, 209 mg/g) | Penicillin G potassium salts hydrolysis 37 °C, 10 cycles | [70] |
| SILLP | Fe3O4–silica hybrid | Imidazolium [Cl]− | CRL | Palm stearin interesterification 45 °C, 4 cycles | [71] |
| SILLP | MWCNTs | Imidazolium [PF6]− | CALB (19,354 U/g, 96 mg/g) | Triacetin hydrolysis 60 °C, 4 cycles | [75] |
| SILLP | MWCNTs | Imidazolium [PF6]− | CALB (25,350 U/g, 114 mg/g) | Triacetin hydrolysis 60 °C, 4 cycles | [76] |
| SILLP | MWCNTs | Imidazolium [PF6]− | CALB (13,636 U/g, 66 mg/g) | Triacetin hydrolysis 60 °C, 4 cycles | [77] |
| SILP | MWCNTs | D-glucose based [NTf2]− | CALB (42 mg/g) | Acrylic acid esterification 25 °C, 5 cycles, Y = 99% 3 | [78] |
| SILLP | MWCNTs | Imidazolium [Oc2PO4]− | CALB (64 mg/g) | 2-adamantanone oxidation 20 °C, 5 cycles, α = 91% 4 | [79] |
| SILP | MWCNTs | Imidazolium [NTf2]− | CALB (22 mg/g) | 2-adamantanone oxidation 20 °C, 4 cycles, α = 99% | [79] |
| SILLP | Silica | Imidazolium [BF4]− | PPL (975 U/mg) | Triacetin hydrolysis 36 °C, 5 cycles | [81] |
| SILLP | Silica | Imidazolium [BF4]− | PPL (975 U/mg) | Triacetin hydrolysis 35 °C, 5 cycles | [82] |
| SILLP | Silica | Imidazolium [BF4]− | BCL (10205 U/g, 230 mg/g) | Triacetin hydrolysis 50 °C, 3 cycles | [83] |
| SILLP | Silica | Imidazolium [BF4]− | PPL (720 U/g, 227.5 mg/g) | Triacetin hydrolysis 35 °C, 4 cycles | [84] |
| SILLP | Silica | Imidazolium L-lysine | PPL (244 U/g, 197 mg/g) | Triacetin hydrolysis 50 °C, 5 cycles | [85] |
| SILLP | Silica | Imidazolium [BF4]− | PPL (392 U/g, 245 mg/g) | Triacetin hydrolysis 50 °C, 5 cycles | [86] |
| SILLP | Silica | Imidazolium [BF4]− | PPL (760 U/g, 117 mg/g) | Triacetin hydrolysis 45 °C, 5 cycles | [87] |
| SILLP | Silica | Imidazolium [BF4]− | PPL (468 U/g, 186 mg/g) | Triacetin hydrolysis 45 °C, 5 cycles | [88] |
| SILLP | Silica | Imidazolium [Cl]− | Papain (0.8 U/mg, 261 mg/g) | L-tyrosine synthesis 50 °C | [89] |
| SILLP | Organosilica | Imidazolium [Cl]− | Amylase from Bacillus amyloliquefaciens (29.35 U/mg, 80 mg/g) | Starch hydrolysis 70 °C, 4 cycles | [90] |
| SILLP | Silica | Imidazolium [BF4]− | CALB (5044.44 U/g) | Corn oil glycerolysis 50 °C, 5 cycles, α = 70.94% | [91] |
| SILP | Silica aerogel | Ammonium [C4H9COO]− | BCL (83% 5) | Olive oil hydrolysis 37 °C, 23 cycles | [92] |
| SILP | Silica aerogel | Ammonium [C4H9COO]− | BCL (337 mg/g) | Coconut oil esterification 40 °C, α = 70% | [93] |
| SILP | Silica | Phosphonium [NTf2]− | BCL (91.1%) | Olive oil hydrolysis 37 °C, 17 cycles | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolny, A.; Chrobok, A. Ionic Liquids for Development of Heterogeneous Catalysts Based on Nanomaterials for Biocatalysis. Nanomaterials 2021, 11, 2030. https://doi.org/10.3390/nano11082030
Wolny A, Chrobok A. Ionic Liquids for Development of Heterogeneous Catalysts Based on Nanomaterials for Biocatalysis. Nanomaterials. 2021; 11(8):2030. https://doi.org/10.3390/nano11082030
Chicago/Turabian StyleWolny, Anna, and Anna Chrobok. 2021. "Ionic Liquids for Development of Heterogeneous Catalysts Based on Nanomaterials for Biocatalysis" Nanomaterials 11, no. 8: 2030. https://doi.org/10.3390/nano11082030