Study of the Molecule Adsorption Process during the Molecular Doping
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Methods
2.2. Computational Methods
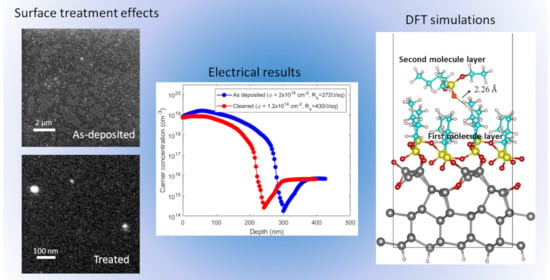
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ho, J.C.; Yerushalmi, R.; Jacobson, Z.A.; Fan, Z.; Alley, R.L.; Javey, A. Controlled nanoscale doping of semiconductors via molecular monolayers. Nat. Mater. 2008, 7, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, R.A.; Garozzo, C.; Bongiorno, C.; Di Franco, S.; Italia, M.; Mannino, G.; Scalese, S.; La Magna, A. Molecular doping applied to Si nanowires array based solar cells. Sol. Energy Mater. Sol. Cells 2015, 132, 118–122. [Google Scholar] [CrossRef]
- Wehling, T.O.; Novoselov, K.S.; Morozov, S.V.; Vdovin, E.E.; Katsnelson, M.I.; Geim, A.K.; Lichtenstein, A.I. Molecular Doping of Graphene. Nano Lett. 2008, 8, 173–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, H.L.; Dull, J.T.; Longhi, E.; Barlow, S.; Rand, B.P.; Marder, S.R.; Kahn, A. n-Doping of a Low-Electron-Affinity Polymer Used as an Electron-Transport Layer in Organic Light-Emitting Diodes. Adv. Funct. Mater. 2020, 30, 2000328. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, E.K.; Shrestha, N.K.; Boukhvalov, D.W.; Lee, J.K.; Han, S.H. Charge Transfer-Induced Molecular Hole Doping into Thin Film of Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2015, 7, 18501–18507. [Google Scholar] [CrossRef] [Green Version]
- Cai, B.; Zhang, S.; Yan, Z.; Zeng, H. Noncovalent Molecular Doping of Two-Dimensional Materials. ChemNanoMat 2015, 1, 542–557. [Google Scholar] [CrossRef]
- Tanigaki, N.; Mochizuki, H.; Mo, X.; Mizokuro, T.; Hiraga, T.; Taima, T.; Yase, K. Molecular doping of poly(p-phenylenevinylene) under vacuum for photovoltaic application. Thin Solid Films 2006, 499, 110–113. [Google Scholar] [CrossRef]
- Arduca, E.; Mastromatteo, M.; Salvador, D.D.; Seguini, G.; Lenardi, C.; Napolitani, E.; Perego, M. Synthesis and characterization of Pδ-layer in SiO2by monolayer doping. Nanotechnology 2016, 27, 75606. [Google Scholar] [CrossRef] [PubMed]
- Voorthuijzen, W.P.; Yilmaz, M.D.; Naber, W.J.M.; Huskens, J.; van der Wiel, W.G. Local Doping of Silicon Using Nanoimprint Lithography and Molecular Monolayers. Adv. Mater. 2011, 23, 1346–1350. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, R.A.; Caccamo, S.; D’Urso, L.; Fisichella, G.; Giannazzo, F.; Italia, M.; La Magna, A. A comprehensive study on the physicochemical and electrical properties of Si doped with the molecular doping method. Phys. Status Solidi A 2015, 212, 1685–1694. [Google Scholar] [CrossRef]
- Alphazan, T.; Mathey, L.; Schwarzwälder, M.; Lin, T.H.; Rossini, A.J.; Wischert, R.; Enyedi, V.; Fontaine, H.; Veillerot, M.; Lesage, A.; et al. Monolayer Doping of Silicon through Grafting a Tailored Molecular Phosphorus Precursor onto Oxide-Passivated Silicon Surfaces. Chem. Mater. 2016, 28, 3634–3640. [Google Scholar] [CrossRef]
- O’Connell, J.; Biswas, S.; Duffy, R.; Holmes, J.D. Chemical approaches for doping nanodevice architectures. Nanotechnology 2016, 27, 342002. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, J.; Verni, G.A.; Gangnaik, A.; Shayesteh, M.; Long, B.; Georgiev, Y.M.; Petkov, N.; McGlacken, G.P.; Morris, M.A.; Duffy, R.; et al. Organo-arsenic Molecular Layers on Silicon for High-Density Doping. ACS Appl. Mater. Interfaces 2015, 7, 15514–15521. [Google Scholar] [CrossRef] [Green Version]
- Garozzo, C.; Bongiorno, C.; Franco, S.D.; Italia, M.; Magna, A.L.; Scalese, S.; Sberna, P.M.; Puglisi, R.A. Nanofabrication processes for innovative nanohole-based solar cells. Phys. Status Solidi A 2013, 210, 1564–1570. [Google Scholar] [CrossRef]
- Veerbeek, J.; Ye, L.; Vijselaar, W.; Kudernac, T.; van der Wiel, W.G.; Huskens, J. Highly doped silicon nanowires by monolayer doping. Nanoscale 2017, 9, 2836–2844. [Google Scholar] [CrossRef] [PubMed]
- Park, C.J.; Jung, S.M.; Kim, J.H.; Shin, M.W. Conformal doping strategy for fin structures: Tailoring of dopant profile through multiple monolayer doping and capping layer control. Semicond. Sci. Technol. 2020, 35, 055028. [Google Scholar] [CrossRef]
- Ang, K.W.; Barnett, J.; Loh, W.Y.; Huang, J.; Min, B.G.; Hung, P.Y.; Ok, I.; Yum, J.H.; Bersuker, G.; Rodgers, M.; et al. 300 mm FinFET results utilizing conformal, damage free, ultra shallow junctions (Xj-5 nm) formed with molecular monolayer doping technique. In Proceedings of the 2011 International Electron Devices Meeting, Washington, DC, USA, 5–7 December 2011; p. 35. [Google Scholar] [CrossRef]
- Ye, L.; Pujari, S.P.; Zuilhof, H.; Kudernac, T.; de Jong, M.P.; van der Wiel, W.G.; Huskens, J. Controlling the Dopant Dose in Silicon by Mixed-Monolayer Doping. ACS Appl. Mater. Interfaces 2015, 7, 3231–3236. [Google Scholar] [CrossRef]
- Caccamo, S.; Puglisi, R.A.; Di Franco, S.; D’Urso, L.; Indelicato, V.; Italia, M.; Pannitteri, S.; La Magna, A. Silicon doped by molecular doping technique: Role of the surface layers of doped Si on the electrical characteristics. Mater. Sci. Semicond. Process. 2016, 42, 200–203. [Google Scholar] [CrossRef]
- Ho, J.C.; Yerushalmi, R.; Smith, G.; Majhi, P.; Bennett, J.; Halim, J.; Faifer, V.N.; Javey, A. Wafer-Scale, Sub-5 nm Junction Formation by Monolayer Doping and Conventional Spike Annealing. Nano Lett. 2009, 9, 725–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, B.; Alessio Verni, G.; O’Connell, J.; Holmes, J.; Shayesteh, M.; O’Connell, D.; Duffy, R. Molecular Layer Doping: Non-destructive doping of silicon and germanium. In Proceedings of the International Conference on Ion Implantation Technology, Portland, OR, USA, 26 June–4 July 2014; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2014. [Google Scholar] [CrossRef] [Green Version]
- Hsu, S.H.; Wan, C.C.; Cho, T.C.; Lee, Y.J. Investigation of Boron Distribution at the SiO2/Si Interface of Monolayer Doping. ACS Omega 2021, 6, 733–738. [Google Scholar] [CrossRef]
- Zhang, C.; Chang, S.; Dan, Y. Advances in ultrashallow doping of silicon. Adv. Phys. X 2021, 6, 1871407. [Google Scholar]
- Garozzo, C.; Giannazzo, F.; Italia, M.; La Magna, A.; Privitera, V.; Puglisi, R.A. Radial junctions formed by conformal chemical doping for innovative hole-based solar cells. Mater. Sci. Eng. B 2013, 178, 686–690. [Google Scholar] [CrossRef]
- Hanson, E.L.; Schwartz, J.; Nickel, B.; Koch, N.; Danisman, M.F. Bonding Self-Assembled, Compact Organophosphonate Monolayers to the Native Oxide Surface of Silicon. J. Am. Chem. Soc. 2003, 125, 16074–16080. [Google Scholar] [CrossRef]
- De Santiago, F.; Trejo, A.; Miranda, A.; Carvajal, E.; Pérez, L.A.; Cruz-Irisson, M. Band-gap engineering of halogenated silicon nanowires through molecular doping. J. Mol. Model. 2017, 23, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Neves, B.R.A.; Salmon, M.E.; Russell, P.E.; Troughton, E.B. Spread Coating of OPA on Mica: From Multilayers to Self-Assembled Monolayers. Langmuir 2001, 17, 8193–8198. [Google Scholar] [CrossRef]
- Ekerdt, J.G.; Klabunde, K.J.; Shapley, J.R.; White, J.M.; Yates, J.T. Surface chemistry of organophosphorus compounds. J. Phys. Chem. 1988, 92, 6182–6188. [Google Scholar] [CrossRef]
- Ho, J.C.; Ford, A.C.; Chueh, Y.L.; Leu, P.W.; Ergen, O.; Takei, K.; Smith, G.; Majhi, P.; Bennett, J.; Javey, A. Nanoscale doping of InAs via sulfur monolayers. Appl. Phys. Lett. 2009, 95, 72108. [Google Scholar] [CrossRef] [Green Version]
- Barnett, J.; Hill, R.; Loh, W.Y.; Hobbs, C.; Majhi, P.; Jammy, R. Advanced techniques for achieving ultra-shallow junctions in future CMOS devices. In Proceedings of the 2010 International Workshop on Junction Technology Extended Abstracts, Shanghai, China, 10–11 May 2010; pp. 1–4. [Google Scholar] [CrossRef]
- Eberhardt, A.; Fenter, P.; Eisenberger, P. Growth kinetics in self-assembling monolayers: A unique adsorption mechanism. Surf. Sci. 1998, 397, L285–L290. [Google Scholar] [CrossRef]
- Doudevski, I.; Schwartz, D.K. Concentration Dependence of Self-Assembled Monolayer Island Nucleation and Growth. J. Am. Chem. Soc. 2001, 123, 6867–6872. [Google Scholar] [CrossRef]
- Doudevski, I.; Hayes, W.A.; Schwartz, D.K. Submonolayer Island Nucleation and Growth Kinetics during Self-Assembled Monolayer Formation. Phys. Rev. Lett. 1998, 81, 4927–4930. [Google Scholar] [CrossRef]
- Silva-Quinones, D.; He, C.; Butera, R.E.; Wang, G.T.; Teplyakov, A.V. Reaction of BCl3 with H- and Cl-terminated Si(100) as a pathway for selective, monolayer doping through wet chemistry. Appl. Surf. Sci. 2020, 533, 146907. [Google Scholar] [CrossRef]
- Nie, H.Y.; Walzak, M.J.; McIntyre, N.S. Bilayer and Odd-Numbered Multilayers of Octadecylphosphonic Acid Formed on a Si Substrate Studied by Atomic Force Microscopy. Langmuir 2002, 18, 2955–2958. [Google Scholar] [CrossRef]
- Gouzman, I.; Dubey, M.; Carolus, M.D.; Schwartz, J.; Bernasek, S.L. Monolayer vs. multilayer self-assembled alkylphosphonate films: X-ray photoelectron spectroscopy studies. Surf. Sci. 2006, 600, 773–781. [Google Scholar] [CrossRef]
- Puglisi, R.A.; Caccamo, S.; Bongiorno, C.; Fisicaro, G.; Genovese, L.; Goedecker, S.; Mannino, G.; La Magna, A. Direct observation of single organic molecules grafted on the surface of a silicon nanowire. Sci. Rep. 2019, 9, 5647. [Google Scholar] [CrossRef] [Green Version]
- Puglisi, R.A.; Nicotra, G.; Lombardo, S.; Spinella, C.; Ammendola, G.; Gerardi, C. Partial self-ordering observed in silicon nanoclusters deposited on silicon oxide substrates by chemical vapor deposition. Phys. Rev. B 2005, 71, 125322. [Google Scholar] [CrossRef]
- Ordejón, P.; Artacho, E.; Soler, J.M. Self-consistent order-N density-functional calculations for very large systems. Phys. Rev. B 1996, 53, R10441–R10444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Kennedy, N.; Garvey, S.; MacCioni, B.; Eaton, L.; Nolan, M.; Duffy, R.; Meaney, F.; Kennedy, M.; Holmes, J.D.; Long, B. Monolayer Doping of Germanium with Arsenic: A New Chemical Route to Achieve Optimal Dopant Activation. Langmuir 2020, 36, 9993–10002. [Google Scholar] [CrossRef]
- Schwartz, D.K. Mechanisms and kinetics of self-assembled monolayer formation. Annu. Rev. Phys. Chem. 2001, 52, 107–137. [Google Scholar] [CrossRef]
- Tu, K.N. Electronic Thin Film Science; Simon & Schuster Books For Young Readers: New York, NY, USA, 1992. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizzone, M.; Grimaldi, M.G.; La Magna, A.; Rahmani, N.; Scalese, S.; Adam, J.; Puglisi, R.A. Study of the Molecule Adsorption Process during the Molecular Doping. Nanomaterials 2021, 11, 1899. https://doi.org/10.3390/nano11081899
Pizzone M, Grimaldi MG, La Magna A, Rahmani N, Scalese S, Adam J, Puglisi RA. Study of the Molecule Adsorption Process during the Molecular Doping. Nanomaterials. 2021; 11(8):1899. https://doi.org/10.3390/nano11081899
Chicago/Turabian StylePizzone, Mattia, Maria Grazia Grimaldi, Antonino La Magna, Neda Rahmani, Silvia Scalese, Jost Adam, and Rosaria A. Puglisi. 2021. "Study of the Molecule Adsorption Process during the Molecular Doping" Nanomaterials 11, no. 8: 1899. https://doi.org/10.3390/nano11081899