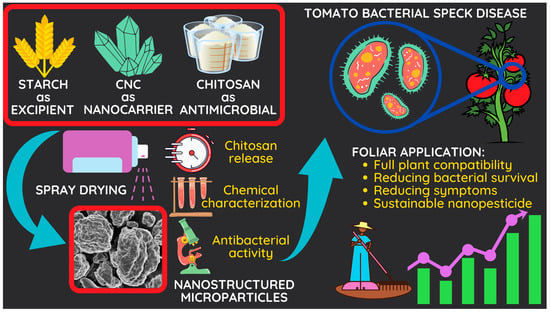
A Green Nanostructured Pesticide to Control Tomato Bacterial Speck Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CNC Synthesis and Characterization
2.2.1. CNC Synthesis
2.2.2. CNC Morphological and Chemical Characterization
2.3. Starch Extraction and Characterization
2.3.1. Starch Extraction
2.3.2. Starch Granule Morphology
2.3.3. Quantification of Amylose, Total Starch and Resistant Starch
2.4. Preliminary In Vitro Antibacterial Activity
Agar Incorporation Assay
2.5. Synthesis and Charachterization of CH-CNC-Starch NMP
2.5.1. Preparation and Characterization of CH-CNC-Starch NMP
2.5.2. CH Quantification Assay
2.5.3. CH Extraction
2.5.4. NMP In Vitro Antibacterial Activity
2.6. CH-CNC-Starch NMP Phytobiological Compatibility
2.7. In Vivo Antibacterial Activity
2.7.1. Bacterial Epiphytic Survival
2.7.2. Disease Symptomatic Expression
2.8. Statistical Analysis
3. Results and Discussion
3.1. CNC Synthesis and Characterization
3.2. ST Extraction and Characterization
3.3. Preliminary In Vitro Antibacterial Activity
3.4. Synthesis and Charachterization of CH-CNC-Starch NMP
3.5. CH-CNC-Starch NMP Phytobiological Compatibility
3.6. In Vivo Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Medina-Pérez, G.; Fernández-Luqueño, F.; Campos-Montiel, R.G.; Sánchez-López, K.B.; Afanador-Barajas, L.N.; Prince, L. Nanotechnology in Crop Protection: Status and Future Trends; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 2018, ISBN 9780128158296. [Google Scholar]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Nanoencapsulation, Nano-guard for Pesticides: A New Window for Safe Application. J. Agric. Food Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in sustainable agriculture: Recent developments, challenges, and perspectives. Front. Microbiol. 2017, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Preston, G.M. Pseudomonas syringae pv. tomato: The right pathogen, of the right plant, at the right time. Mol. Plant Pathol. 2000, 1, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Babelegoto, N.; Varvaro, L.; Cirulli, M. Epiphytic, and endophytic multiplication of Pseudomonas syringae pv. tomato (Okabel) Young et al. in susceptible and resistant tomato leaves. Phytopathol. Mediterr. 1988, 27, 138–144. [Google Scholar]
- McCarter, S.M. Survival of Pseudomonas syringae pv. tomato in Association with Tomato Seed, Soil, Host Tissue, and Epiphytic Weed Hosts in Georgia. Phytopathology 1983, 73, 1393. [Google Scholar] [CrossRef]
- Griffin, K.; Gambley, C.; Brown, P.; Li, Y. Copper-tolerance in Pseudomonas syringae pv. tomato and Xanthomonas spp. and the control of diseases associated with these pathogens in tomato and pepper. A systematic literature review. Crop Prot. 2017, 96. [Google Scholar] [CrossRef]
- Abrahamian, P.; Jones, J.B.; Vallad, G.E. Efficacy of copper and copper alternatives for management of bacterial spot on tomato under transplant and field production. Crop Prot. 2019, 126. [Google Scholar] [CrossRef]
- Obradovic, A.; Jones, J.B.; Momol, M.T.; Balogh, B.; Olson, S.M. Management of tomato bacterial spot in the field by foliar applications of bacteriophages and SAR inducers. Plant Dis. 2004, 88, 736–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Torre, A.; Iovino, V.; Caradonia, F. Copper in plant protection: Current situation and prospects. Phytopathol. Mediterr. 2018, 57, 201–236. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J.B.; Aubertot, J.N. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sustain. Dev. 2018, 38, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Flemming, C.A.; Trevors, J.T. Copper toxicity and chemistry in the environment: A review. Water Air Soil Pollut. 1989, 44, 143–158. [Google Scholar] [CrossRef]
- Keller, A.A.; Adeleye, A.S.; Conway, J.R.; Garner, K.L.; Zhao, L.; Cherr, G.N.; Hong, J.; Gardea-Torresdey, J.L.; Godwin, H.A.; Hanna, S.; et al. Comparative environmental fate and toxicity of copper nanomaterials. Nano Impact 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Bender, C.L.; Cooksey, D.A. Indigenous plasmids in Pseudomonas syringae pv. tomato: Conjugative transfer and role in copper resistance. J. Bacteriol. 1986, 165, 534–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLeod, A.; Masimba, T.; Jensen, T.; Serfontein, K.; Coertze, S. Evaluating spray programs for managing copper resistant Pseudomonas syringae pv. tomato populations on tomato in the Limpopo region of South Africa. Crop Prot. 2017, 102, 32–42. [Google Scholar] [CrossRef]
- Quattrucci, A.; Ovidi, E.; Tiezzi, A.; Vinciguerra, V.; Balestra, G.M. Biological control of tomato bacterial speck using Punica granatum fruit peel extract. Crop Prot. 2013. [Google Scholar] [CrossRef]
- Vargas, P.; Farias, G.A.; Nogales, J.; Prada, H.; Carvajal, V.; Barón, M.; Rivilla, R.; Martín, M.; Olmedilla, A.; Gallegos, M.T. Plant flavonoids target Pseudomonas syringae pv. tomato DC3000 flagella and type III secretion system. Environ. Microbiol. Rep. 2013, 5, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Hernández, J.; Arredondo-Valdés, R.; Reyes-Zepeda, F.; Hernández-Castillo, F.D.; Anguiano-Cabello, J.C.; Heinz-Castro, R.T.Q.; Mora-Ravelo, S.G. In vitro antibacterial activity of Magnolia tamaulipana against tomato phytopathogenic bacteria. Plant Prot. Sci. 2020, 56, 1–7. [Google Scholar] [CrossRef]
- Sabir, A.; El-Khalfi, B.; Errachidi, F.; Chemsi, I.; Serrano, A.; Soukri, A. Evaluation of the Potential of Some Essential Oils in Biological Control against Phytopathogenic Agent Pseudomonas syringae pv. Tomato DC3000 Responsible for the Tomatoes Speck. J. Plant Pathol. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Li, K.; Xing, R.; Liu, S.; Li, P. Chitin and Chitosan Fragments Responsible for Plant Elicitor and Growth Stimulator. J. Agric. Food Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- De Bona, G.S.; Vincenzi, S.; De Marchi, F.; Angelini, E.; Bertazzon, N. Chitosan induces delayed grapevine defense mechanisms and protects grapevine against Botrytis cinerea. J. Plant Dis. Prot. 2021, 128, 715–724. [Google Scholar] [CrossRef]
- Al-Tawaha, A.R.M.; Al-Ghzawi, A.L.A. Effect of chitosan coating on seed germination and salt tolerance of Lentil (Lens culinaris L.). Res. Crop 2013, 14, 489–491. [Google Scholar]
- Ziani, K.; Ursúa, B.; Maté, J.I. Application of bioactive coatings based on chitosan for artichoke seed protection. Crop Prot. 2010, 29, 853–859. [Google Scholar] [CrossRef]
- Betchem, G.; Johnson, N.A.N.; Wang, Y. The application of chitosan in the control of post-harvest diseases: A review. J. Plant Dis. Prot. 2019, 126, 495–507. [Google Scholar] [CrossRef]
- Scortichini, M. Field efficacy of chitosan to control Pseudomonas syringae pv. actinidiae, the causal agent of kiwifruit bacterial canker. Eur. J. Plant Pathol. 2014, 140, 887–892. [Google Scholar] [CrossRef]
- Pichyangkura, R.; Chadchawan, S. Biostimulant activity of chitosan in horticulture. Sci. Hortic. Amst. 2015, 196, 49–65. [Google Scholar] [CrossRef]
- Hemantaranjan, A. A Future Perspective in Crop Protection: Chitosan and its Oligosaccharides. Adv. Plants Agric. Res. 2014, 1, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hassan, O.; Chang, T. Chitosan for Eco-friendly Control of Plant Disease. Asian J. Plant Pathol. 2017, 11, 53–70. [Google Scholar] [CrossRef]
- Borines, L.; Sagarino, R.; Calamba, R.; Contioso, M.A.; Jansalin, J.G.; Calibo, C. Potential of Chitosan for the Control of Tomato Bacterial Wilt Caused by Ralstonia solanacearum (Smith) Yabuuchi et al. Ann. Trop. Res. 2015, 57–69. [Google Scholar] [CrossRef] [Green Version]
- Benhamou, N.; Kloepper, J.W.; Tuzun, S. Induction of resistance against Fusarium wilt of tomato by combination of chitosan with an endophytic bacterial strain: Ultrastructure and cytochemistry of the host response. Planta 1998, 204, 153–168. [Google Scholar] [CrossRef]
- El Amerany, F.; Meddich, A.; Wahbi, S.; Porzel, A.; Taourirte, M.; Rhazi, M.; Hause, B. Foliar application of chitosan increases tomato growth and influences mycorrhization and expression of endochitinase-encoding genes. Int. J. Mol. Sci. 2020, 21, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jail, N.G.; Luiz, C.; Da Rocha Neto, A.C.; Di Piero, R.M. High-density chitosan reduces the severity of bacterial spot and activates the defense mechanisms of tomato plants. Trop. Plant Pathol. 2014, 39, 434–441. [Google Scholar] [CrossRef] [Green Version]
- Mansilla, A.Y.; Albertengo, L.; Rodríguez, M.S.; Debbaudt, A.; Zúñiga, A.; Casalongué, C.A. Evidence on antimicrobial properties and mode of action of a chitosan obtained from crustacean exoskeletons on Pseudomonas syringae pv. tomato DC3000. Appl. Microbiol. Biotechnol. 2013, 97, 6957–6966. [Google Scholar] [CrossRef]
- Signini, R.; Campana Filho, S.P. On the preparation and characterization of chitosan hydrochloride. Polym. Bull. 1999, 42, 159–166. [Google Scholar] [CrossRef]
- Francesconi, S.; Steiner, B.; Buerstmayr, H.; Lemmens, M.; Sulyok, M.; Balestra, G.M. Chitosan hydrochloride decreases fusarium graminearum growth and virulence and boosts growth, development and systemic acquired resistance in two durum wheat genotypes. Molecules 2020, 25, 4752. [Google Scholar] [CrossRef]
- Fortunati, E.; Giovanale, G.; Luzi, F.; Mazzaglia, A.; Kenny, J.M.; Torre, L.; Balestra, G.M. Effective postharvest preservation of kiwifruit and romaine lettuce with a chitosan hydrochloride coating. Coatings 2017, 7, 196. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Aly, A.; Kim, K. Nano-based smart pesticide formulations: Emerging opportunities for agriculture. J. Control. Release 2019, 294, 131–153. [Google Scholar] [CrossRef]
- Alejandro, P.D.L.; Rubiales, D. Nanotechnology for parasitic plant control. Pest Manag. Sci. 2009, 65, 540–545. [Google Scholar] [CrossRef]
- Elsharkawy, M.; Derbalah, A.; Hamza, A.; El-Shaer, A. Zinc oxide nanostructures as a control strategy of bacterial speck of tomato caused by Pseudomonas syringae in Egypt. Environ. Sci. Pollut. Res. 2020, 27, 19049–19057. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, D.; Cui, J. Graphene oxide loaded with copper oxide nanoparticles as an antibacterial agent against: Pseudomonas syringae pv. tomato. RSC Adv. 2017, 7, 38853–38860. [Google Scholar] [CrossRef] [Green Version]
- Ocsoy, I.; Paret, M.L.; Ocsoy, M.A.; Kunwar, S.; Chen, T.; You, M.; Tan, W. Nanotechnology in plant disease management: DNA-directed silver nanoparticles on graphene oxide as an antibacterial against Xanthomonas perforans. ACS Nano 2013, 7, 8972–8980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Shetehy, M.; Moradi, A.; Maceroni, M.; Reinhardt, D.; Petri-Fink, A.; Rothen-Rutishauser, B.; Mauch, F.; Schwab, F. Silica nanoparticles enhance disease resistance in Arabidopsis plants. Nat. Nanotechnol. 2021, 16, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, A.; Young, M.; Lee, B.; Liao, Y.Y.; Da Silva, S.; Godden, D.; Colee, J.; Huang, Z.; Mendis, H.C.; Campos, M.G.N.; et al. Copper-fixed quat: A hybrid nanoparticle for application as a locally systemic pesticide (LSP) to manage bacterial spot disease of tomato. Nanoscale Adv. 2021, 3, 1473–1483. [Google Scholar] [CrossRef]
- Fortunati, E.; Verma, D.; Luzi, F.; Mazzaglia, A.; Torre, L.; Balestra, G.M. Novel nanoscaled materials from lignocellulosic sources: Potential applications in the agricultural sector. Handb. Ecomater. 2019, 4, 2657–2679. [Google Scholar] [CrossRef]
- Fortunati, E.; Mazzaglia, A.; Balestra, G.M. Sustainable control strategies for plant protection and food packaging sectors by natural substances and novel nanotechnological approaches. J. Sci. Food Agric. 2019, 99, 986–1000. [Google Scholar] [CrossRef]
- Campos, E.V.R.; de Oliveira, J.L.; Fraceto, L.F.; Singh, B. Polysaccharides as safer release systems for agrochemicals. Agron. Sustain. Dev. 2015, 35, 47–66. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Roman, M. Toxicity of cellulose nanocrystals: A review. Ind. Biotechnol. 2015, 11, 25–33. [Google Scholar] [CrossRef]
- Salas, C.; Nypelö, T.; Rodriguez-Abreu, C.; Carrillo, C.; Rojas, O.J. Nanocellulose properties and applications in colloids and interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 383–396. [Google Scholar] [CrossRef]
- Kamel, R.; El-Wakil, N.A.; Dufresne, A.; Elkasabgy, N.A. Nanocellulose: From an agricultural waste to a valuable pharmaceutical ingredient. Int. J. Biol. Macromol. 2020, 163, 1579–1590. [Google Scholar] [CrossRef]
- Cortesi, R.; Quattrucci, A.; Esposito, E.; Mazzaglia, A.; Balestra, G.M. Natural antimicrobials in spray-dried microparticles based on cellulose derivatives as potential eco-compatible agrochemicals. J. Plant Dis. Prot. 2017, 124, 269–278. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Puglia, D. Synergic effect of cellulose and lignin nanostructures in PLA based systems for food antibacterial packaging. Eur. Polym. J. 2016, 79, 1–12. [Google Scholar] [CrossRef]
- Fortunati, E.; Rescignano, N.; Botticella, E.; La Fiandra, D.; Renzi, M.; Mazzaglia, A.; Torre, L.; Kenny, J.M.; Balestra, G.M. Effect of poly(DL-lactide-co-glycolide) nanoparticles or cellulose nanocrystals-based formulations on Pseudomonas syringae pv. tomato (Pst) and tomato plant development. J. Plant Dis. Prot. 2016, 123, 301–310. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Falsafi, S.R.; Jafari, S.M. Starch-based nanocarriers as cutting-edge natural cargos for nutraceutical delivery. Trends Food Sci. Technol. 2019, 88, 397–415. [Google Scholar] [CrossRef]
- Dureja, H.; Khatakb, S.; Khatakc, M.K. Amylose Rich Starch as an Aqueous Based Pharmaceutical Coating Material—Review. Int. J. Pharm. Sci. Drug Res. 2011, 3, 8–12. [Google Scholar]
- Liu, Z.; Han, J.H. Film-forming Characteristics of Starches. J. Food Sci. 2005, 70, 31–36. [Google Scholar]
- Feltre, G.; Almeida, F.S.; Sato, A.C.K.; Dacanal, G.C.; Hubinger, M.D. Alginate and corn starch mixed gels: Effect of gelatinization and amylose content on the properties and in vitro digestibility. Food Res. Int. 2020, 132, 109069. [Google Scholar] [CrossRef]
- Hong, Y.; Liu, G.; Gu, Z. Recent advances of starch-based excipients used in extended-release tablets: A review. Drug Deliv. 2016, 23, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, S.; Nugent, A.P. Health effects of resistant starch. Nutr. Bull. 2017, 42, 10–41. [Google Scholar] [CrossRef]
- Botticella, E.; Sestili, F.; Sparla, F.; Moscatello, S.; Marri, L.; Cuesta-Seijo, J.A.; Falini, G.; Battistelli, A.; Trost, P.; Lafiandra, D. Combining mutations at genes encoding key enzymes involved in starch synthesis affects the amylose content, carbohydrate allocation and hardness in the wheat grain. Plant Biotechnol. J. 2018, 16, 1723–1734. [Google Scholar] [CrossRef] [Green Version]
- Cranston, E.D.; Gray, D.G. Morphological and optical characterization of polyelectrolyte multilayers incorporating nanocrystalline cellulose. Biomacromolecules 2006, 7, 2522–2530. [Google Scholar] [CrossRef] [PubMed]
- Sparla, F.; Falini, G.; Botticella, E.; Pirone, C.; Talamè, V.; Bovina, R.; Salvi, S.; Tuberosa, R.; Sestili, F.; Trost, P. New starch phenotypes produced by TILLING in barley. PLoS ONE 2014, 9, e107779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrastil, J. Improved colorimetric determination of amylose in starches or flours. Carbohydr. Res. 1987, 159, 154–158. [Google Scholar] [CrossRef]
- Cazorla, F.M.; Arrebola, E.; Sesma, A.; Pérez-García, A.; Codina, J.C.; Murillo, J.; De Vicente, A. Copper resistance in Pseudomonas syringae strains isolated from mango is encoded mainly by plasmids. Phytopathology 2002, 92, 909–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, E.O.; Ward, M.K.; Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 1954. [Google Scholar] [CrossRef]
- Uppalapati, S.R.; Ishiga, Y.; Wangdi, T.; Urbanczyk-Wochniak, E.; Ishiga, T.; Mysore, K.S.; Bender, C.L. Pathogenicity of Pseudomonas syringae pv. tomato on tomato seedlings: Phenotypic and gene expression analyses of the virulence function of coronatine. Mol. Plant Microbe Interact. 2008, 21, 383–395. [Google Scholar] [CrossRef] [Green Version]
- Canzoniere, P.; Francesconi, S.; Giovando, S.; Balestra, G.M. Antibacterial activity of tannins towards Pseudomonas syringae pv. tomato, and their potential as biostimulants on tomato plants. Phytopathol. Mediterr. 2021, 60, 23–36. [Google Scholar] [CrossRef]
- Mojumdar, A.; Upadhyay, A.K.; Raina, V.; Ray, L. A simple and rapid colorimetric method for the estimation of chitosan produced by microbial degradation of chitin waste. J. Microbiol. Methods 2019, 158, 66–70. [Google Scholar] [CrossRef]
- Zgoda, J.R.; Porter, J.R. A convenient microdilution method for screening natural products against bacteria and fungi. Pharm. Biol. 2001, 39, 221–225. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Barchiesi, F.; Del Prete, M.S.; Fortuna, M.; Caselli, F.; Scalise, G. In vitro susceptibility tests for cationic peptides: Comparison of broth microdilution methods for bacteria that grow aerobically. Antimicrob. Agents Chemother. 2000, 44, 1694–1696. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.-C.; Guo, G.-J.; Guo, Y.-M. Measuring Plant Leaf Area by Scanner and ImageJ Software. China Veg. 2011, 1, 73–77. [Google Scholar]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef]
- Li, B.; Shan, C.L.; Ge, M.Y.; Wang, L.; Fang, Y.; Wang, Y.L.; Xie, G.L.; Sun, G.C. Antibacterial mechanism of chitosan and its applications in protection of plant from bacterial disease. Asian J. Chem. 2013, 25, 10033–10036. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs. 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.R.; Mihu, M.R.; Han, G.; Frases, S.; Cordero, J.B.; Casadevall, A.; Friedman, A.J.; Friedman, J.M.; Nosanchuk, J.D. The Use of Chitosan to Damage Cryptococcus. Biomaterials. 2010, 31, 669–679. [Google Scholar] [CrossRef] [Green Version]
- Pasquantonio, G.; Greco, C.; Prenna, M.; Ripa, C.; Vitali, L.A.; Petrelli, D.; Di Luca, M.C.; Ripa, S. Antibacterial activity and anti-biofilm effect of chitosan against strains of Streptococcus mutans isolated in dental plaque. Int. J. Immunopathol. Pharmacol. 2008, 21, 993–997. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.; Pham, D.T.N.; Oloketuyi, S.F.; Manivasagan, P.; Oh, J.; Kim, Y.M. Chitosan and their derivatives: Antibiofilm drugs against pathogenic bacteria. Colloids Surf. B Biointerfaces 2020, 185, 110627. [Google Scholar] [CrossRef]
- Goy, R.C.; De Britto, D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polimeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Malveau, C.; Baille, W.E.; Zhu, X.X.; Marchessault, R.H. NMR imaging of high-amylose starch tablets. 2. Effect of tablet size. Biomacromolecules 2002, 3, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Onofre, F.; Wang, Y.J.; Mauromoustakos, A. Effects of structure and modification on sustained release properties of starches. Carbohydr. Polym. 2009, 76, 541–547. [Google Scholar] [CrossRef]
- Xin, X.F.; Kvitko, B.; He, S.Y. Pseudomonas syringae: What it takes to be a pathogen. Nat. Rev. Microbiol. 2018, 16, 316–328. [Google Scholar] [CrossRef]
- Rouse, D.I.; Nordheim, E.V.; Hirano, S.S.; Upper, C.D. A Model Relating the Probability of Foliar Disease Incidence to the Population Frequencies of Bacterial Plant Pathogens. Phytopathology 1985, 75, 505. [Google Scholar] [CrossRef]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in plant protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, D.; Hemantaranjan, A.; Singh, B. Chitosan as a promising natural compound to enhance potential physiological responses in plant: A review. Indian J. Plant Physiol. 2015, 20, 1–9. [Google Scholar] [CrossRef]
- Amborabé, B.E.; Bonmort, J.; Fleurat-Lessard, P.; Roblin, G. Early events induced by chitosan on plant cells. J. Exp. Bot. 2008, 59, 2317–2324. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Genotype | Total Starch (%) | Resistant Starch (%) | Amylose (%) |
|---|---|---|---|
| Cadenza | 58.9 ± 0.1 a | 0.8 ± 0.2 a | 32.0 ± 0.5 a |
| Cad-SBEIIa | 57 ± 0.9 a | 6.9 ± 0.4 b | 63.1 ± 2.9 b |
| CH-CNC-HA Starch NMP | ||||||
|---|---|---|---|---|---|---|
| Sample | Powder (g) | Chitosan (g) | CC (% w/w) | %RSD | Yield | |
| Replicates | Mean ± SD | |||||
| A1 | 0.0041 | 0.00176 | 43.0 | 45.1 ± 2.9 | 6.4 | 52% |
| A2 | 0.0042 | 0.00184 | 43.9 | |||
| A3 | 0.0045 | 0.00222 | 49.3 | |||
| A4 | 0.0045 | 0.00199 | 44.2 | |||
| CH-CNC-St Starch NMP | ||||||
| Sample | Powder (g) | Chitosan (g) | CC (% w/w) | %RSD | Yield | |
| Replicates | Mean ± SD | |||||
| B1 | 0.0039 | 0.00167 | 42.8 | 46.7 ± 3.5 | 7.5 | 55% |
| B2 | 0.0041 | 0.00196 | 47.8 | |||
| B3 | 0.0040 | 0.00198 | 49.5 | |||
| Treatment | Biological Parameters | Days Post Treatment | ||
|---|---|---|---|---|
| 1 dpt | 7 dpt | 14 dpt | ||
| Water | Leaf Area (cm2) | 3.8 ± 0.1 | 4.0 ± 0.2 | 7.1 ± 0.7 |
| Chlorophyll Content (DU) | 27.6 ± 1.0 | 26.9 ± 1.9 | 28.0 ± 0.6 | |
| Flavonols Content (DU) | 0.56 ± 0.04 | 0.39 ± 0.01 | 0.41 ± 0.02 | |
| Nitrogen Balance Index | 59.3 ± 3.5 | 71.2 ± 5.1 | 71.4 ± 2.5 | |
| Copper Hydroxide | Leaf Area (cm2) | 3.4 ± 0.1 | 5.6 ± 0.5 | 7.8 ± 1.2 |
| Chlorophyll Content (DU) | 27.8 ± 1.3 | 29.4 ± 1.0 | 28.2 ± 0.8 | |
| Flavonols Content (DU) | 0.74 ± 0.06 | 0.44 ± 0.03 | 0.38 ± 0.01 | |
| Nitrogen Balance Index | 45.1 ± 4.4 | 71.2 ± 3.4 | 75.6 ± 2.3 | |
| CH-CNC-Standard starch NMP | Leaf Area (cm2) | 3.7 ± 0.2 | 5.0 ± 0.6 | 7.3 ± 0.8 |
| Chlorophyll Content (DU) | 26.2 ± 1.1 | 26.1 ± 0.6 | 28.6 ± 1.0 | |
| Flavonols Content (DU) | 0.58 ± 0.04 | 0.45 ± 0.03 | 0.38 ± 0.01 | |
| Nitrogen Balance Index | 50.9 ± 3.6 | 63.0 ± 2.9 | 75.8 ± 2.8 | |
| CH -CNC-High amylose NMP | Leaf Area (cm2) | 3.3 ± 0.1 | 4.9 ± 0.7 | 7.7 ± 0.7 |
| Chlorophyll Content (DU) | 27.6 ± 0.9 | 28.1 ± 1.6 | 28.7 ± 0.5 | |
| Flavonols Content (DU) | 0.69 ± 0.06 | 0.42 ± 0.03 | 0.46 ± 0.03 | |
| Nitrogen Balance Index | 46.2 ± 3.0 | 69.4 ± 2.9 | 67.1 ± 3.4 | |
| Treatment | 7 Days Post Inoculation (p < 0.01) | |
|---|---|---|
| DS (Necrosis Per Plant) | DR (%) | |
| Water | 38.1 ± 4.9 a | - |
| Copper Hydroxide | 25.1 ± 2.9 ab | 34.1 ± 7.9 |
| CH-CNC-HA Starch NMP | 20.6 ± 2.8 b | 49.9 ± 7.5 |
| CH-CNC-St Starch NMP | 19.1 ± 3.7 b | 45.9 ± 9.6 |
| Treatment | Disease Incidence (%) (p < 0.01) | |
|---|---|---|
| 7 dpi | 14 dpi | |
| Water | 4.8 ± 0.2 a | 4.9 ± 0.3 a |
| Copper Hydroxide | 2.3 ± 0.2 b | 3.5 ± 0.4 b |
| CH-CNC-HA Starch NMP | 3 ± 0.4 b | 3 ± 0.2 b |
| CH-CNC-St Starch NMP | 3 ± 0.2 b | 2.7 ± 0.04 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiavi, D.; Balbi, R.; Giovagnoli, S.; Camaioni, E.; Botticella, E.; Sestili, F.; Balestra, G.M. A Green Nanostructured Pesticide to Control Tomato Bacterial Speck Disease. Nanomaterials 2021, 11, 1852. https://doi.org/10.3390/nano11071852
Schiavi D, Balbi R, Giovagnoli S, Camaioni E, Botticella E, Sestili F, Balestra GM. A Green Nanostructured Pesticide to Control Tomato Bacterial Speck Disease. Nanomaterials. 2021; 11(7):1852. https://doi.org/10.3390/nano11071852
Chicago/Turabian StyleSchiavi, Daniele, Rosa Balbi, Stefano Giovagnoli, Emidio Camaioni, Ermelinda Botticella, Francesco Sestili, and Giorgio Mariano Balestra. 2021. "A Green Nanostructured Pesticide to Control Tomato Bacterial Speck Disease" Nanomaterials 11, no. 7: 1852. https://doi.org/10.3390/nano11071852