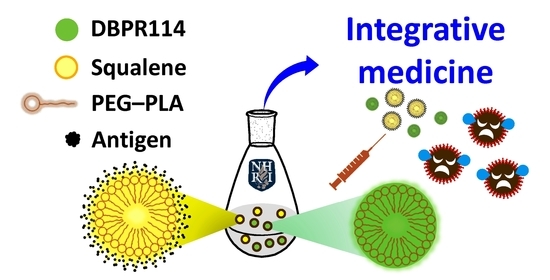
Colloidal Assemblies Composed of Polymeric Micellar/Emulsified Systems Integrate Cancer Therapy Combining a Tumor-Associated Antigen Vaccine and Chemotherapeutic Regimens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
2.3. Cell Viability Assay
2.4. Ethic Statement
2.5. Integrative Medicine
3. Results
3.1. Characterization of PEG–PLA by NMR, GPC and Aqueous Solubilization
3.2. Anti-Proliferative Activity of DBPR114 in Leukemia Cell Lines
3.3. Co-Delivery of Antitumor Drug/Antigen Agents Enhances Antitumor Efficacy
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vanneman, M.; Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 2012, 12, 237–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.T.; Phung, C.D.; Tran, T.H.; Pham, T.T.; Pham, L.M.; Nguyen, T.T.; Jeong, J.H.; Choi, H.G.; Ku, S.K.; Yong, C.S.; et al. Manipulating immune system using nanoparticles for an effective cancer treatment: Combination of targeted therapy and checkpoint blockage miRNA. J. Control. Release 2021, 329, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tai, X.; Shi, K.; Ruan, S.; Qiu, Y.; Zhang, Z.; Xiang, B.; He, Q. A new concept of enhancing immuno-chemotherapeutic effects against B16F10 tumor via systemic administration by taking advantages of the limitation of EPR effect. Theranostics 2016, 6, 2141–2160. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Liu, S.J.; Leng, C.H.; Chen, H.W.; Chong, P. Disintegration and cancer immunotherapy efficacy of a squalane-in-water delivery system emulsified by bioresorbable poly(ethylene glycol)-block-polylactide. Biomaterials 2014, 35, 1686–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.Y.; Huang, C.H.; Liu, S.J.; Chen, H.W.; Leng, C.H.; Chong, P.; Huang, M.H. Polysorbasome: A colloidal vesicle contoured by polymeric bioresorbable amphiphiles as an immunogenic depot for vaccine delivery. ACS Appl. Mater. Interfaces 2018, 10, 12553–12561. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Huang, C.Y.; Ho, H.M.; Lee, C.H.; Lai, P.T.; Wu, S.C.; Liu, S.J.; Huang, M.H. Nanoemulsion adjuvantation strategy of tumor-associated antigen therapy rephrases mucosal and immunotherapeutic signatures following intranasal vaccination. J. Immunother. Cancer 2020, 8, e001022. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Coumar, M.S.; Wang, W.C.; Shiao, H.Y.; Ke, Y.Y.; Lin, W.H.; Kuo, C.C.; Chang, C.W.; Kuo, F.M.; Chen, P.Y.; et al. Discovery of BPR1K871, a quinazoline based, multi-kinase inhibitor for the treatment of AML and solid tumors: Rational design, synthesis, in vitro and in vivo evaluation. Oncotarget 2016, 7, 86239–86256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, M.T.; Ganguly, S.; Wang, T.L.; Anderson, C.W.; Sadtler, K.; Narain, R.; Cherry, C.; Parrillo, A.J.; Park, B.V.; Wang, G.; et al. A biologic scaffold-associated type 2 immune microenvironment inhibits tumor formation and synergizes with checkpoint immunotherapy. Sci. Transl. Med. 2019, 11, eaat7973. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.E.; Caenepeel, S.; Wu, L.C. Targeted therapy and checkpoint immunotherapy combinations for the treatment of cancer. Trends Immunol. 2016, 37, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Gotwals, P.; Cameron, S.; Cipolletta, D.; Cremasco, V.; Crystal, A.; Hewes, B.; Mueller, B.; Quaratino, S.; Sabatos-Peyton, C.; Petruzzelli, L.; et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat. Rev. Cancer 2017, 17, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramakrishnan, R.; Assudani, D.; Nagaraj, S.; Hunter, T.; Cho, H.I.; Antonia, S.; Altiok, S.; Celis, E.; Gabrilovich, D.I. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J. Clin. Investig. 2010, 120, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Chang, J.Y. New insights into mechanisms of cisplatin resistance: From tumor cell to microenvironment. Int. J. Mol. Sci. 2019, 20, 4136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, L.; Liu, Q.; Shen, L.; Liu, Y.; Zhang, X.; Chen, F.; Huang, L. Nano-delivery of fraxinellone remodels tumor microenvironment and facilitates therapeutic vaccination in desmoplastic melanoma. Theranostics 2018, 8, 3781–3796. [Google Scholar] [CrossRef] [PubMed]


| Sample | 1H NMR a) | GPC b) | Particle Analysis in Water c) | |||||
|---|---|---|---|---|---|---|---|---|
| Size (nm) | Number (kcps) | |||||||
| WPEG/WPLA | Mn | Mn | Mw/Mn | Blank | With DBPR114 | Blank | With DBPR114 | |
| P1 | 50:50 | 4000 | 4500 | 1.23 | 60 ± 10 | 50 ± 10 | 1400 ± 85 | 1050 ± 240 |
| P2 | 70:30 | 2800 | 3100 | 1.12 | 185 ± 60 | 5950 ± 1100 | 450 ± 30 | 320 ± 40 |
| Cell Line | EC50 (nM) a) | ||
|---|---|---|---|
| Formulations | MOLM-13 (Heterozygous) | K562 (Control Group) | |
| P1 | >50,000 | >50,000 | |
| P2 | >80,000 | >80,000 | |
| DBPR114/DMSO | 1.05 | 7620 | |
| DBPR114/water | 10 | 9821 | |
| DBPR114/P1 | 1.6 | 4615 | |
| DBPR114/P2 | 1.5 | 7110 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-Y.; Lin, S.-Y.; Hsu, T.-A.; Hsieh, H.-P.; Huang, M.-H. Colloidal Assemblies Composed of Polymeric Micellar/Emulsified Systems Integrate Cancer Therapy Combining a Tumor-Associated Antigen Vaccine and Chemotherapeutic Regimens. Nanomaterials 2021, 11, 1844. https://doi.org/10.3390/nano11071844
Huang C-Y, Lin S-Y, Hsu T-A, Hsieh H-P, Huang M-H. Colloidal Assemblies Composed of Polymeric Micellar/Emulsified Systems Integrate Cancer Therapy Combining a Tumor-Associated Antigen Vaccine and Chemotherapeutic Regimens. Nanomaterials. 2021; 11(7):1844. https://doi.org/10.3390/nano11071844
Chicago/Turabian StyleHuang, Chiung-Yi, Shu-Yu Lin, Tsu-An Hsu, Hsing-Pang Hsieh, and Ming-Hsi Huang. 2021. "Colloidal Assemblies Composed of Polymeric Micellar/Emulsified Systems Integrate Cancer Therapy Combining a Tumor-Associated Antigen Vaccine and Chemotherapeutic Regimens" Nanomaterials 11, no. 7: 1844. https://doi.org/10.3390/nano11071844