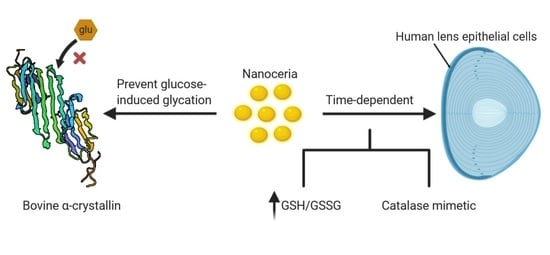
Nanoceria Prevents Glucose-Induced Protein Glycation in Eye Lens Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticles Synthesis and Characterisation
2.2. Cell Culture
2.3. Time-Dependent Uptake of EGCNPs in HLECs (ICP-MS Studies)
2.4. Mechanism of EGCNPs Uptake in HLECs
2.5. Catalase-Mimetic Activity of EGCNPs in HLECs
2.6. Effect of EGCNPs on GSH/GSSG Ratio
2.7. Measuring Antiglycation Properties of EGCNPs on BSA and Bovine α-Crystallin
2.8. Statistical Analysis
3. Results and Discussion
3.1. EGCNPs Characterisation
3.2. Time-Dependent Uptake of EGCNPs in HLECs and Its Mechanism
3.3. In Vitro Catalase-Like Activity of EGCNPs in HLECs
3.4. Effect of EGCNPs on GSH/GSSG Ratio
3.5. Effect of EGCNPs on Protein Glycation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harding, J.J. Viewing molecular mechanisms of ageing through a lens. Ageing Res. Rev. 2002, 1, 465–479. [Google Scholar] [CrossRef]
- Vinson, J.A. Oxidative stress in cataracts. Pathophysiology 2006, 13, 151–162. [Google Scholar] [CrossRef]
- Toh, T.Y.; Morton, J.; Coxon, J.; Elder, M.J. Medical treatment of cataract. Clin. Exp. Ophthalmol. 2007, 35, 664–671. [Google Scholar] [CrossRef]
- Truscott, R.J.W. Age-related nuclear cataract–Oxidation is the key. Exp. Eye Res. 2005, 80, 709–725. [Google Scholar] [CrossRef]
- Klein, B.E.K.; Klein, R.; Linton, K.L.P. Prevalence of Age-related Lens Opacities in a Population: The Beaver Dam Eye Study. Ophthalmology 1992, 99, 546–552. [Google Scholar] [CrossRef]
- Thrimawithana, T.R.; Rupenthal, I.D.; Räsch, S.S.; Lim, J.C.; Morton, J.D.; Bunt, C.R. Drug delivery to the lens for the management of cataracts. Adv. Drug Deliv. Rev. 2018, 126, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Moreau, K.L.; King, J.A. Protein misfolding and aggregation in cataract disease and prospects for prevention. Trends Mol. Med. 2012, 18, 273–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truscott, R.J.W.; Friedrich, M.G. The etiology of human age-related cataract. Proteins don’t last forever. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Hook, D.W.A.; Harding, J.J. Protection of enzymes by α-crystallin acting as a molecular chaperone. Int. J. Biol. Macromol. 1998, 22, 295–306. [Google Scholar] [CrossRef]
- Cobb, B.A.; Petrash, J.M. α-crystallin chaperone-like activity and membrane binding in age-related cataracts. Biochemistry 2002, 41, 483–490. [Google Scholar] [CrossRef]
- Andley, U.P. The lens epithelium: Focus on the expression and function of the α-crystallin chaperones. Int. J. Biochem. Cell Biol. 2008, 40, 317–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berthoud, V.M.; Beyer, E.C. Oxidative stress, lens gap junctions, and cataracts. Antioxidants Redox Signal. 2009, 11, 339–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pescosolido, N.; Barbato, A.; Giannotti, R.; Komaiha, C.; Lenarduzzi, F. Age-related changes in the kinetics of human lenses: Prevention of the cataract. Int. J. Ophthalmol. 2016, 9, 1506–1517. [Google Scholar] [PubMed]
- Brian, G.; Taylor, H. Cataract blindness–Challenges for the 21st century. Bull. World Health Organ. 2001, 79, 249–256. [Google Scholar] [PubMed]
- Xu, C.; Qu, X. Cerium oxide nanoparticle: A remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014, 6, e90. [Google Scholar] [CrossRef]
- Li, C.; Shi, X.; Shen, Q.; Guo, C.; Hou, Z.; Zhang, J. Hot Topics and Challenges of Regenerative Nanoceria in Application of Antioxidant Therapy. J. Nanomater. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Mullins, D.R. The surface chemistry of cerium oxide. Surf. Sci. Rep. 2015, 70, 42–85. [Google Scholar] [CrossRef] [Green Version]
- Plakhova, T.V.; Romanchuk, A.Y.; Butorin, S.M.; Konyukhova, A.D.; Egorov, A.V.; Shiryaev, A.A.; Baranchikov, A.E.; Dorovatovskii, P.V.; Huthwelker, T.; Gerber, E.; et al. Towards the surface hydroxyl species in CeO2 nanoparticles. Nanoscale 2019, 11, 18142–18149. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S. Redox-dependent catalase mimetic cerium oxide-based nanozyme protect human hepatic cells from 3-AT induced acatalasemia. Colloids Surfaces B Biointerfaces 2019, 175, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Korsvik, C.; Patil, S.; Seal, S.; Self, W.T. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem. Commun. 2007, 2007, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, B.I.; Cave, G.W.V.; Barnett, Y.; Pierscionek, B. Ethylene glycol coated nanoceria protects against oxidative stress in human lens epithelium. RSC Adv. 2019, 9, 16596–16605. [Google Scholar] [CrossRef] [Green Version]
- Hanafy, B.I.; Cave, G.W.V.; Barnett, Y.; Pierscionek, B. Treatment of Human Lens Epithelium with High Levels of Nanoceria Leads to Reactive Oxygen Species Mediated Apoptosis. Molescules 2020, 25, 441. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Ojeda, F.J.; Gomez-Llorente, C.; Aguilera, C.M.; Gil, A.; Rupérez, A.I. Impact of 3-Amino-1,2,4-Triazole (3-AT)-Derived Increase in Hydrogen Peroxide Levels on Inflammation and Metabolism in Human Differentiated Adipocytes. PLoS ONE 2016, 11, e0152550. [Google Scholar] [CrossRef] [Green Version]
- Abdelkader, H.; Longman, M.; Alany, R.G.; Pierscionek, B. On the Anticataractogenic Effects of L-Carnosine: Is It Best Described as an Antioxidant, Metal-Chelating Agent or Glycation Inhibitor? Oxidative Med. Cell. Longev. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Gräfe, C.; Weidner, A.; Lühe, M.V.D.; Bergemann, C.; Schacher, F.H.; Clement, J.H.; Dutz, S. Intentional formation of a protein corona on nanoparticles: Serum concentration affects protein corona mass, surface charge, and nanoparticle-cell interaction. Int. J. Biochem. Cell Biol. 2016, 75, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Ly, A.; Das, S.; Sakthivel, T.S.; Barkam, S.; Seal, S. Cerium oxide nanoparticles at the nano-bio interface: Size-dependent cellular uptake. Artif. Cells Nanomed. Biotechnol. 2018, 46, S956–S963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leroueil, P.R.; Berry, S.A.; Duthie, K.; Han, G.; Rotello, V.M.; McNerny, D.Q.; Baker, J.R.; Orr, B.G.; Holl, M.M.B. Wide varieties of cationic nanoparticles induce defects in supported lipid bilayers. Nano Lett. 2008, 8, 420–424. [Google Scholar] [CrossRef]
- Kostarelos, K.; Lacerda, L.; Pastorin, G.; Wu, W.; Wieckowski, S.; Luangsivilay, J.; Godefroy, S.; Pantarotto, D.; Briand, J.P.; Muller, S.; et al. Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nat. Nanotechnol. 2007, 2, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, A.; Karakoti, A.; Seal, S.; Self, W.T. Unveiling the mechanism of uptake and sub-cellular distribution of cerium oxide nanoparticles. Mol. Biosyst. 2010, 6, 1813–1820. [Google Scholar] [CrossRef] [Green Version]
- Letoha, T.; Keller-Pintér, A.; Kusz, E.; Kolozsi, C.; Bozsó, Z.; Tóth, G.; Vizler, C.; Oláh, Z.; Szilák, L. Cell-penetrating peptide exploited syndecans. Biochim. Biophys. Acta Biomembr. 2010, 1798, 2258–2265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asati, A.; Santra, S.; Kaittanis, C.; Perez, J.M. Surface-charge-dependent cell localization and cytotoxicity of cerium oxide nanoparticles. ACS Nano 2010, 4, 5321–5331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Liu, Y.; Ge, J.; Wang, X.; Liu, L.; Bu, Z.; Liu, P. Resveratrol protects human lens epithelial cells against H2O2-induced oxidative stress by increasing catalase, SOD-1, and HO-1 expression. Mol. Vis. 2010, 16, 1467–1474. [Google Scholar]
- Wolf, N.; Penn, P.; Pendergrass, W.; Van Remmen, H.; Bartke, A.; Rabinovitch, P.; Martin, G.M. Age-related cataract progression in five mouse models for anti-oxidant protection or hormonal influence. Exp. Eye Res. 2005, 81, 276–285. [Google Scholar] [CrossRef]
- Ganea, E.; Harding, J.J. Glutathione-related enzymes and the eye. Curr. Eye Res. 2006, 31, 1–11. [Google Scholar] [CrossRef]
- Yang, J.; Gong, X.; Fang, L.; Fan, Q.; Cai, L.; Qiu, X.; Zhang, B.; Chang, J.; Lu, Y. Potential of CeCl3@mSiO2 nanoparticles in alleviating diabetic cataract development and progression. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef] [Green Version]
- Beswick, H.T.; Harding, J.J. Conformational changes induced in lens alpha- and gamma-crystallins by modification with glucose 6-phosphate. Implications for cataract. Biochem. J. 1987, 246, 761–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, A.; Schmitt, J.; Münch, G.; Gasic-Milencovic, J. Characterization of advanced glycation end products for biochemical studies: Side chain modifications and fluorescence characteristics. Anal. Biochem. 2005, 338, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Leclère, J.; Birlouez-Aragon, I. The Fluorescence of Advanced Maillard Products Is a Good Indicator of Lysine Damage during the Maillard Reaction. J. Agric. Food Chem. 2001, 49, 4682–4687. [Google Scholar] [CrossRef]
- Jedziniak, J.A.; Chylack, L.T.; Cheng, H.M.; Gillis, M.K.; Kalustian, A.A.; Tung, W.H. The sorbitol pathway in the human lens: Aldose reductase and polyol dehydrogenase. Investig. Ophthalmol. Vis. Sci. 1981, 20, 314–326. [Google Scholar]
- Blakytny, R.; Harding, J.J. Prevention of the inactivation of glutathione reductase by fructation using human α-crystallin. Biochem. Soc. Trans. 1995, 23, 610S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blakytny, R.; Harding, J.J. Prevention of the fructation-lnduced inactivation of glutathione reductase by bovine α-crystallin acting as a molecular chaperone. Ophthalmic Res. 1996, 28, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, J.E.; Dotzel, E.; Clark, A.F. Steroid-lnduced cataract: New perspectives from in vitro and lens culture studies. Exp. Eye Res. 1997, 65, 507–516. [Google Scholar] [CrossRef]
- Hook, D.W.A.; Harding, J.J. Alpha-crystallin acting as a molecular chaperone protects catalase against steroid-induced inactivation. FEBS Lett. 1996, 18, 281–284. [Google Scholar] [CrossRef] [Green Version]
- Swamy-Mruthinti, S.; Green, K.; Abraham, E.C. Inhibition of cataracts in moderately diabetic rats by aminoguanidine. Exp. Eye Res. 1996, 62, 505–510. [Google Scholar] [CrossRef]
- Agardh, E.; Hultberg, B.; Agardh, C.D. Effects of inhibition of glycation and oxidative stress on the development of cataract and retinal vessel abnormalities in diabetic rats. Curr. Eye Res. 2000, 21, 543–549. [Google Scholar] [CrossRef]
- Greiling, T.M.S.; Clark, J.I. New Insights into the Mechanism of Lens Development Using Zebra Fish. In International Review of Cell and Molecular Biology; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 296, pp. 1–61. [Google Scholar]
- Yang, J.; Cai, L.; Zhang, S.; Zhu, X.; Zhou, P.; Lu, Y. Silica-based cerium (III) chloride nanoparticles prevent the fructose-induced glycation of α-crystallin and H2O2-induced oxidative stress in human lens epithelial cells. Arch. Pharm. Res. 2014, 37, 404–411. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanafy, B.I.; Cave, G.W.V.; Barnett, Y.; Pierscionek, B.K. Nanoceria Prevents Glucose-Induced Protein Glycation in Eye Lens Cells. Nanomaterials 2021, 11, 1473. https://doi.org/10.3390/nano11061473
Hanafy BI, Cave GWV, Barnett Y, Pierscionek BK. Nanoceria Prevents Glucose-Induced Protein Glycation in Eye Lens Cells. Nanomaterials. 2021; 11(6):1473. https://doi.org/10.3390/nano11061473
Chicago/Turabian StyleHanafy, Belal I., Gareth W. V. Cave, Yvonne Barnett, and Barbara K. Pierscionek. 2021. "Nanoceria Prevents Glucose-Induced Protein Glycation in Eye Lens Cells" Nanomaterials 11, no. 6: 1473. https://doi.org/10.3390/nano11061473