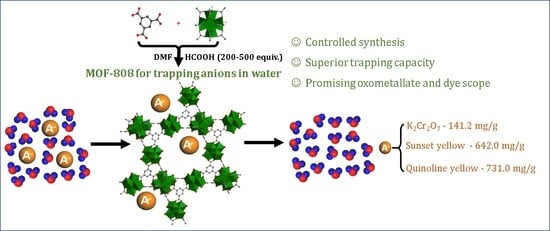
Efficient Removal of Chromium(VI) Anionic Species and Dye Anions from Water Using MOF-808 Materials Synthesized with the Assistance of Formic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of MOF-808 Analogs
2.2. Adsorption Studies
2.2.1. Cr2O72− adsorption
2.2.2. Dye Adsorption
2.3. Instruments
3. Results and Discussion
3.1. MOF-808 Anologues Synthesis and Characterization
3.2. Adsorption Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Wang, B.; Cao, Y.; Zhao, S.; Wang, H.; Feng, X.; Zhou, J.; Ma, X. Water Contaminant Elimination Based on Metal–Organic Frameworks and Perspective on Their Industrial Applications. ACS Sustain. Chem. Eng. 2019, 7, 4548–4563. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, J.; Cai, J.; Zhang, L.; Cui, Y.; Yang, Y.; Chen, B.; Qian, G. A porous Zr-cluster-based cationic metal–organic framework for highly efficient Cr2O72− removal from water. Chem. Commun. 2015, 51, 14732–14734. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yao, S.; Yu, C.; Li, G.; Liu, C.; Huo, Q.; Liu, Y. An ultrastable Zr-MOF for fast capture and highly luminescence detection of Cr2O72− simultaneously in an aqueous phase. J. Mater. Chem. A 2018, 6, 6363–6369. [Google Scholar] [CrossRef]
- Lin, Z.-J.; Zheng, H.-Q.; Zeng, Y.-N.; Wang, Y.-L.; Chen, J.; Cao, G.-J.; Gu, J.-F.; Chen, B. Effective and selective adsorption of organoarsenic acids from water over a Zr-based metal-organic framework. Chem. Eng. J. 2019, 378, 122196. [Google Scholar] [CrossRef]
- DeCoste, J.B.; Peterson, G.W. Metal–Organic Frameworks for Air Purification of Toxic Chemicals. Chem. Rev. 2014, 114, 5695–5727. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kim, J.; Malgras, V.; Na, J.; Lin, J.; You, J.; Zhang, M.; Li, J.; Yamauchi, Y. Water Purification: Metal–Organic Frameworks and Their Derived Materials: Emerging Catalysts for a Sulfate Radicals-Based Advanced Oxidation Process in Water Purification (Small 16/2019). Small 2019, 15, 1970085. [Google Scholar] [CrossRef] [Green Version]
- Jang, E.-H.; Pack, S.P.; Kim, I.; Chung, S. A systematic study of hexavalent chromium adsorption and removal from aqueous environments using chemically functionalized amorphous and mesoporous silica nanoparticles. Sci. Rep. 2020, 10, 5558. [Google Scholar] [CrossRef]
- Sykam, N.; Jayram, N.D.; Rao, G.M. Highly efficient removal of toxic organic dyes, chemical solvents and oils by mesoporous exfoliated graphite: Synthesis and mechanism. J. Water Process. Eng. 2018, 25, 128–137. [Google Scholar] [CrossRef]
- Pakade, V.E.; Tavengwa, N.T.; Madikizela, L.M. Recent advances in hexavalent chromium removal from aqueous solutions by adsorptive methods. RSC Adv. 2019, 9, 26142–26164. [Google Scholar] [CrossRef] [Green Version]
- Jarrah, A.; Farhadi, S. Preparation and characterization of novel polyoxometalate/CoFe2O4/metal–organic framework magnetic core–shell nanocomposites for the rapid removal of organic dyes from water. RSC Adv. 2020, 10, 39881–39893. [Google Scholar] [CrossRef]
- Pincus, L.N.; Rudel, H.E.; Petrović, P.V.; Gupta, S.; Westerhoff, P.; Muhich, C.L. and Zimmerman, J.B. Exploring the Mechanisms of Selectivity for Environmentally Significant Oxo-Anion Removal during Water Treatment: A Review of Common Competing Oxo-Anions and Tools for Quantifying Selective Adsorption. Environ. Sci. Technol. 2020, 54, 9769–9790. [Google Scholar] [CrossRef] [PubMed]
- Sule, R.; Mishra, A.K. MOFs-carbon hybrid nanocomposites in environmental protection applications. Environ. Sci. Pollut. Res. 2020, 27, 16004–16018. [Google Scholar] [CrossRef] [PubMed]
- Shahnawaz Khan, M.; Khalid, M.; Shahid, M. What triggers dye adsorption by metal organic frameworks? The current perspectives. Mater. Adv. 2020, 1, 1575–1601. [Google Scholar] [CrossRef]
- Dias, E.M.; Petit, C. Towards the use of metal–organic frameworks for water reuse: A review of the recent advances in the field of organic pollutants removal and degradation and the next steps in the field. J. Mater. Chem. A. 2015, 3, 22484–22506. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Liu, J.; Wei, F.; Zhu, Y.; Wang, X.; Cao, C.; Song, W. Size-selective adsorption of anionic dyes induced by the layer space in layered double hydroxide hollow microspheres. Mater. Chem. Front. 2017, 1, 1550–1555. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.; Xie, L.-H.; Rutledge, W.; Li, J.-R.; Zhou, H.-C. Zr-based metal–organic frameworks: design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef]
- Evans, J.D.; Garai, B.; Reinsch, H.; Li, W.; Dissegna, S.; Bon, V.; Senkovska, I.; Fischer, R.A.; Kaskel, S.; Janiak, C.; et al. Metal–organic frameworks in Germany: From synthesis to function. Coord. Chem. Rev. 2019, 380, 378–418. [Google Scholar] [CrossRef] [Green Version]
- Drache, F.; Cirujano, F.G.; Nguyen, K.D.; Bon, V.; Senkovska, I.; Llabrés i Xamena, F.X.; Kaskel, S. Anion Exchange and Catalytic Functionalization of the Zirconium-Based Metal–Organic Framework DUT-67. Cryst. Growth Des. 2018, 18, 5492–5500. [Google Scholar] [CrossRef]
- Nguyen, K.D.; Kutzscher, C.; Ehrling, S.; Senkovska, I.; Bon, V.; de Oliveira, M.; Gutmann, T.; Buntkowsky, G.; Kaskel, S. Insights into the role of zirconium in proline functionalized metal-organic frameworks attaining high enantio- and diastereoselectivity. J. Catal. 2019, 377, 41–50. [Google Scholar] [CrossRef]
- He, T.; Zhang, Y.-Z.; Kong, X.-J.; Yu, J.; Lv, X.-L.; Wu, Y.; Guo, Z.-J.; Li, J.-R. Zr(IV)-Based Metal-Organic Framework with T-Shaped Ligand: Unique Structure, High Stability, Selective Detection, and Rapid Adsorption of Cr2O72– in Water. ACS Appl. Mater. Interfaces 2018, 10, 16650–16659. [Google Scholar] [CrossRef]
- Hashem, T.; Ibrahim, A.H.; Wöll, C.; Alkordi, M.H. Grafting Zirconium-Based Metal–Organic Framework UiO-66-NH2 Nanoparticles on Cellulose Fibers for the Removal of Cr(VI) Ions and Methyl Orange from Water. ACS Appl. Nano Mater. 2019, 2, 5804–5808. [Google Scholar] [CrossRef]
- Feng, X.; Hajek, J.; Jena, H.S.; Wang, G.; Veerapandian, S.K.P.; Morent, R.; De Geyter, N.; Leyssens, K.; Hoffman, A.E.J.; Meynen, V.; et al. Engineering a Highly Defective Stable UiO-66 with Tunable Lewis- Brønsted Acidity: The Role of the Hemilabile Linker. J. Am. Chem. Soc. 2020, 142, 3174–3183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saiz, P.G.; Iglesias, N.; González Navarrete, B.; Rosales, M.; Quintero, Y.M.; Reizabal, A.; Orive, J.; Fidalgo Marijuan, A.; Larrea, E.S.; Lopes, A.C.; et al. Chromium Speciation in Zirconium-Based Metal–Organic Frameworks for Environmental Remediation. Chem. Eur. J. 2020, 26, 13861–13872. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.D.; Kutzscher, C.; Drache, F.; Senkovska, I.; Kaskel, S. Chiral Functionalization of a Zirconium Metal–Organic Framework (DUT-67) as a Heterogeneous Catalyst in Asymmetric Michael Addition Reaction. Inorg. Chem. 2018, 57, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Gándara, F.; Zhang, Y.-B.; Jiang, J.; Queen, W.L.; Hudson, M.R.; Yaghi, O.M. Water Adsorption in Porous Metal–Organic Frameworks and Related Materials. J. Am. Chem. Soc. 2014, 136, 4369–4381. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Gándara, F.; Zhang, Y.-B.; Na, K.; Yaghi, O.M.; Klemperer, W.G. Superacidity in Sulfated Metal–Organic Framework-808. J. Am. Chem. Soc. 2014, 136, 12844–12847. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hanna, S.L.; Redfern, L.R.; Alezi, D.; Islamoglu, T.; Farha, O.K. Reticular chemistry in the rational synthesis of functional zirconium cluster-based MOFs. Coord. Chem. Rev. 2019, 386, 32–49. [Google Scholar] [CrossRef]
- Ho, P.H.; Salles, F.; Di Renzo, F.; Trens, P. One-pot synthesis of 5-FU@ZIF-8 and ibuprofen@ZIF-8 nanoparticles. Inorganica Chim. Acta. 2020, 500, 119229. [Google Scholar] [CrossRef]
- Bon, V.; Senkovska, I.; Weiss, M.S.; Kaskel, S. Tailoring of network dimensionality and porosity adjustment in Zr- and Hf-based MOFs. CrystEngComm 2013, 15, 9572–9577. [Google Scholar] [CrossRef]
- Drache, F.; Bon, V.; Senkovska, I.; Marschelke, C.; Synytska, A.; Kaskel, S. Postsynthetic Inner-Surface Functionalization of the Highly Stable Zirconium-Based Metal–Organic Framework DUT-67. Inorg. Chem. 2016, 55, 7206–7213. [Google Scholar] [CrossRef]
- Krause, S.; Bon, V.; Du, H.; Dunin-Borkowski, R.E.; Stoeck, U.; Senkovska, I.; Kaskel, S. The impact of crystal size and temperature on the adsorption-induced flexibility of the Zr-based metal–organic framework DUT-98. Beilstein J. Nanotechnol. 2019, 10, 1737–1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.T.L.; Nguyen, T.T.; Nguyen, K.D.; Phan, N.T.S. Metal–organic framework MOF-199 as an efficient heterogeneous catalyst for the aza-Michael reaction. Appl. Catal. A Gen. 2012, 425-426, 44–52. [Google Scholar] [CrossRef]
- Fu, H.-R.; Xu, Z.-X.; Zhang, J. Water-Stable Metal–Organic Frameworks for Fast and High Dichromate Trapping via Single-Crystal-to-Single-Crystal Ion Exchange. Chem. Mater. 2015, 27, 205–210. [Google Scholar] [CrossRef]
- Chen, S.-S.; Li, C.-W.; Hsu, H.-D.; Lee, P.-C.; Chang, Y.-M.; Yang, C.-H. Concentration and purification of chromate from electroplating wastewater by two-stage electrodialysis processes. J. Hazard. Mater. 2009, 161, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Brito, F.; Ascanio, J.; Mateo, S.; Hernández, C.; Araujo, L.; Gili, P.; Martín-Zarza, P.; Domínguez, S.; Mederos, A. Equilibria of chromate(VI) species in acid medium and ab initio studies of these species. Polyhedron 1997, 16, 3835–3846. [Google Scholar] [CrossRef]
- Caratelli, C.; Hajek, J.; Cirujano, F.G.; Waroquier, M.; Llabrés i Xamena, F.X.; Van Speybroeck, V. Nature of active sites on UiO-66 and beneficial influence of water in the catalysis of Fischer esterification. J. Catal. 2017, 352, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Parida, K.; Mishra, K.G.; Dash, S.K. Adsorption of toxic metal ion Cr(VI) from aqueous state by TiO2-MCM-41: Equilibrium and kinetic studies. J. Hazard. Mater. 2012, 241–242, 395–403. [Google Scholar] [CrossRef]
- Kumar, P.; Saini, M.; Kumar, V.; Singh, M.; Dehiya, B.S.; Umar, A.; Ajmal Khan, M.; Alhuwaymel, T.F. Removal of Cr (VI) from aqueous solution using VO2(B) nanoparticles. Chem. Phys. Lett. 2020, 739, 136934. [Google Scholar] [CrossRef]
- Zeng, H.; Zeng, H.; Zhang, H.; Shahab, A.; Zhang, K.; Lu, Y.; Nabi, I.; Naseem, F.; Ullah, H. Efficient adsorption of Cr (VI) from aqueous environments by phosphoric acid activated eucalyptus biochar. J. Clean. Prod. 2021, 286, 124964. [Google Scholar] [CrossRef]
- Aliabadi, M.; Khazaei, I.; Fakhraee, H.; Mousavian, M.T.H. Hexavalent chromium removal from aqueous solutions by using low-cost biological wastes: Equilibrium and kinetic studies. Int. J. Environ. Sci. Technol. (Tehran) 2012, 9, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Mostafazadeh, N.; Ghoreyshi, A.A.; Pirzadeh, K. Optimization of solvothermally synthesized ZIF-67 metal organic framework and its application for Cr(VI) adsorption from aqueous solution. Iran. J. Chem. Chem. Eng. 2018, 15, 27–47. [Google Scholar]
- Guo, J.; Li, J.-J.; Wang, C.-C. Adsorptive removal of Cr(VI) from simulated wastewater in MOF BUC-17 ultrafine powder. J. Environ. Chem. Eng. 2019, 7, 102909. [Google Scholar] [CrossRef]
- Suraj Prakash, T.; Satyabrata, S.; Rashmi, A.; Kulamani, P.; Raghunath, A.; Mira, D. Adsorptive removal of Cr(VI) onto UiO-66-NH2 and its determination by radioanalytical techniques. J. Radioanal. Nucl. Chem. 2019, 322, 983–992. [Google Scholar]
- Rapti, S.; Pournara, A.; Sarma, D.; Papadas, I.T.; Armatas, G.S.; Tsipis, A.C.; Lazarides, T.; Kanatzidis, M.G.; Manos, M.J. Selective capture of hexavalent chromium from an anion-exchange column of metal organic resin–alginic acid composite. Chem. Sci. 2016, 7, 2427–2436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Ye, Y.; Sun, X.; Liu, B.; Li, G.; Liang, Z.; Liu, Y. A multifunctional Zr(iv)-based metal–organic framework for highly efficient elimination of Cr(vi) from the aqueous phase. J. Mater. Chem. A 2019, 7, 16833–16841. [Google Scholar] [CrossRef]
- Azad, F.N.; Ghaedi, M.; Dashtian, K.; Hajati, S.; Pezeshkpour, V. Ultrasonically assisted hydrothermal synthesis of activated carbon–HKUST-1-MOF hybrid for efficient simultaneous ultrasound-assisted removal of ternary organic dyes and antibacterial investigation: Taguchi optimization. Ultrason. Sonochem. 2016, 31, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shi, Z.; Zhu, H.; Hong, W.; Xie, F.; Sun, K. Adsorption of azo dyes from aqueous solution by the hybrid MOFs/GO. Water Sci. Technol. 2016, 73, 1728–1737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Askari, H.; Ghaedi, M.; Dashtian, K.; Azghandi, M.H.A. Rapid and high-capacity ultrasonic assisted adsorption of ternary toxic anionic dyes onto MOF-5-activated carbon: Artificial neural networks, partial least squares, desirability function and isotherm and kinetic study. Ultrason. Sonochem. 2017, 37, 71–82. [Google Scholar] [CrossRef]
- Kökçam-Demir, Ü.; Goldman, A.; Esrafili, L.; Gharib, M.; Morsali, A.; Weingart, O.; Janiak, C. Coordinatively unsaturated metal sites (open metal sites) in metal–organic frameworks: Design and applications. Chem. Soc. Rev. 2020, 49, 2751–2798. [Google Scholar] [CrossRef]
















| No. | Sample Name | Formic Acid Amount (Equivalents) | Volume of Formic Acid (mL) | Volume of DMF (mL) | Mass of Product (g) | Yield of Reaction (%) |
|---|---|---|---|---|---|---|
| 1 | MOF-808_200 | 200 | 19 | 56 | 0.34 | 55 |
| 2 | MOF-808_250 | 250 | 24 | 51 | 0.47 | 76 |
| 3 | MOF-808_300 | 300 | 28 | 47 | 0.51 | 83 |
| 4 | MOF-808_350 | 350 | 33 | 42 | 0.45 | 73 |
| 5 | MOF-808_400 | 400 | 38 | 37 | 0.42 | 68 |
| 6 | MOF-808_450 | 450 | 43 | 32 | 0.36 | 59 |
| 7 | MOF-808_500 | 500 | 47 | 28 | 0.22 | 36 |
| No. | Sample Name | Formic Acid Amount (Equivalent) | Cell Parameter 1 (Å) | Crystal Diameter 2 (nm) | BET Surface Area (m2/g) | Average Pore Diameter 3 (Å) | V Total Pore 3 (cm3/g) | V Micropore 3 (cm3/g) |
|---|---|---|---|---|---|---|---|---|
| 1 | MOF-808_200 | 200 | 35.12 ± 0.04 | <40 | 2370 | 23.9 | 1.18 | 0.59 |
| 2 | MOF-808_250 | 250 | 35.15 ± 0.02 | ~60 | 1627 | 21.9 | 0.80 | 0.44 |
| 3 | MOF-808_300 | 300 | 35.27 ± 0.01 | ~300 | 3291 | 18.7 | 0.82 | 0.79 |
| 4 | MOF-808_350 | 350 | 35.19 ± 0.05 | ~600 | 1464 | 19.1 | 0.41 | 0.4 |
| 5 | MOF-808_400 | 400 | 35.22 ± 0.02 | ~700 | 1853 | 18.7 | 0.51 | 0.50 |
| 6 | MOF-808_450 | 450 | 35.30 ± 0.02 | ~1000 | 2279 | 18.6 | 0.60 | 0.60 |
| 7 | MOF-808_500 | 500 | 35.31 ± 0.05 | ~1000 | 2677 | 17.8 | 0.63 | 0.62 |
| No. | Sample Name | K2Cr2O7 Capacity (mg/g) | Sunset Yellow Capacity (mg/g) | Quinoline Yellow Capacity (mg/g) | Ref. |
|---|---|---|---|---|---|
| 1 | UiO-66 with 15% missing-linker defects | 8.8 | - | - | [23] |
| 2 | UiO-66 with 25% missing-linker defects | 22.4 | [23] | ||
| 3 | UiO-66-NH2 | 34.4 | - | - | [23] |
| 4 | UiO-66-(OH)2 | 75.5 | - | [23] | |
| 5 | UiO-66-HA | 129.0 | - | [44] | |
| 6 | UiO-66-NH2@SiO2 | 137.0 | - | - | [21] |
| 7 | JLU—MOF60 | 149.0 | - | - | [45] |
| 8 | Mesoporous silica nanoparticles | 42.2 | - | - | [7] |
| 9 | MOF-199 | - | - | 65.4 | [46] |
| 10 | MIL-101@graphene oxide | - | 81.3 | - | [47] |
| 11 | MOF-5@activated carbon | - | - | 21.2 | [48] |
| 12 | Activated carbon | No adsorption | 96.0 | 97.0 | This work |
| 13 | MOF-808 | 78.0–141.2 | 366.0–642.0 | 659.9–731.0 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, K.D.; Ho, P.H.; Vu, P.D.; Pham, T.L.D.; Trens, P.; Di Renzo, F.; Phan, N.T.S.; Le, H.V. Efficient Removal of Chromium(VI) Anionic Species and Dye Anions from Water Using MOF-808 Materials Synthesized with the Assistance of Formic Acid. Nanomaterials 2021, 11, 1398. https://doi.org/10.3390/nano11061398
Nguyen KD, Ho PH, Vu PD, Pham TLD, Trens P, Di Renzo F, Phan NTS, Le HV. Efficient Removal of Chromium(VI) Anionic Species and Dye Anions from Water Using MOF-808 Materials Synthesized with the Assistance of Formic Acid. Nanomaterials. 2021; 11(6):1398. https://doi.org/10.3390/nano11061398
Chicago/Turabian StyleNguyen, Khoa D., Phuoc H. Ho, Phuong D. Vu, Thuyet L. D. Pham, Philippe Trens, Francesco Di Renzo, Nam T. S. Phan, and Ha V. Le. 2021. "Efficient Removal of Chromium(VI) Anionic Species and Dye Anions from Water Using MOF-808 Materials Synthesized with the Assistance of Formic Acid" Nanomaterials 11, no. 6: 1398. https://doi.org/10.3390/nano11061398