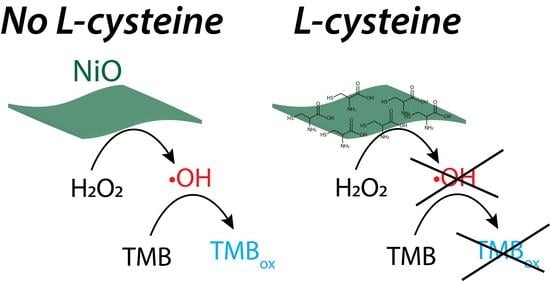
L-Cysteine as an Irreversible Inhibitor of the Peroxidase-Mimic Catalytic Activity of 2-Dimensional Ni-Based Nanozymes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of β-Ni(OH)2 and NiO
2.3. Material Characterization
2.4. Peroxidase-Mimic Catalytic Activity of Nanozymes and Optimization
2.5. Interaction of L-Cysteine Amino Acid with NiO Nanozyme
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breslow, R. Artificial enzymes. Science 1982, 218, 532–537. [Google Scholar] [CrossRef] [Green Version]
- Breslow, R. Biomimetic chemistry and artificial enzymes: Catalysis by design. Acc. Chem. Res. 1995, 28, 146–153. [Google Scholar] [CrossRef]
- Kuah, E.; Toh, S.; Yee, J.; Ma, Q.; Gao, Z. Enzyme Mimics: Advances and Applications. Chemistry 2016, 22, 8404–8430. [Google Scholar] [CrossRef]
- Karim, M.N.; Singh, M.; Weerathunge, P.; Bian, P.; Zheng, R.; Dekiwadia, C.; Ahmed, T.; Walia, S.; Della Gaspera, E.; Singh, S. Visible-light-triggered reactive-oxygen-species-mediated antibacterial activity of peroxidase-mimic CuO nanorods. ACS Appl. Nano Mater. 2018, 1, 1694–1704. [Google Scholar] [CrossRef]
- Weerathunge, P.; Behera, B.K.; Zihara, S.; Singh, M.; Prasad, S.N.; Hashmi, S.; Mariathomas, P.R.D.; Bansal, V.; Ramanathan, R. Dynamic interactions between peroxidase-mimic silver NanoZymes and chlorpyrifos-specific aptamers enable highly-specific pesticide sensing in river water. Anal. Chim. Acta. 2019, 1083, 157–165. [Google Scholar] [CrossRef]
- Weerathunge, P.; Ramanathan, R.; Shukla, R.; Sharma, T.K.; Bansal, V. Aptamer-controlled reversible inhibition of gold nanozyme activity for pesticide sensing. Anal. Chem. 2014, 86, 11937–11941. [Google Scholar] [CrossRef]
- Weerathunge, P.; Ramanathan, R.; Torok, V.A.; Hodgson, K.; Xu, Y.; Goodacre, R.; Behera, B.K.; Bansal, V. Ultrasensitive colorimetric detection of murine norovirus using NanoZyme aptasensor. Anal. Chem. 2019, 91, 3270–3276. [Google Scholar] [CrossRef]
- Karim, M.N.; Anderson, S.R.; Singh, S.; Ramanathan, R.; Bansal, V. Nanostructured silver fabric as a free-standing NanoZyme for colorimetric detection of glucose in urine. Biosens. Bioelectron. 2018, 110, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.K.; Ramanathan, R.; Weerathunge, P.; Mohammadtaheri, M.; Daima, H.K.; Shukla, R.; Bansal, V. Aptamer-mediated ‘turn-off/turn-on’nanozyme activity of gold nanoparticles for kanamycin detection. Chem. Commun. 2014, 50, 15856–15859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weerathunge, P.; Pooja, D.; Singh, M.; Kulhari, H.; Mayes, E.L.; Bansal, V.; Ramanathan, R. Transferrin-conjugated quasi-cubic SPIONs for cellular receptor profiling and detection of brain cancer. Sens. Actuat. B 2019, 297, 126737. [Google Scholar] [CrossRef]
- Naveen Prasad, S.; Weerathunge, P.; Karim, M.N.; Anderson, S.; Hashmi, S.; Mariathomas, P.D.; Bansal, V.; Ramanathan, R. Non-invasive detection of glucose in human urine using a color-generating copper NanoZyme. Anal. Bioanal. Chem. 2021, 413, 1279–1291. [Google Scholar] [CrossRef]
- Das, R.; Dhiman, A.; Kapil, A.; Bansal, V.; Sharma, T.K. Aptamer-mediated colorimetric and electrochemical detection of Pseudomonas aeruginosa utilizing peroxidase-mimic activity of gold NanoZyme. Anal. Bioanal. Chem. 2019, 411, 1229–1238. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef] [PubMed]
- Lien, C.W.; Chen, Y.C.; Chang, H.T.; Huang, C.C. Logical regulation of the enzyme-like activity of gold nanoparticles by using heavy metal ions. Nanoscale 2013, 5, 8227–8234. [Google Scholar] [CrossRef]
- Tseng, C.-W.; Chang, H.-Y.; Chang, J.-Y.; Huang, C.-C. Detection of mercury ions based on mercury-induced switching of enzyme-like activity of platinum/gold nanoparticles. Nanoscale 2012, 4, 6823–6830. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-H.; Chu, L.; Liu, W.; Jiang, L.; Chen, X.-Y.; Wang, Y.-H.; Zhao, Y.-L. The screening of metal ion inhibitors for glucose oxidase based on the peroxidase-like activity of nano-Fe3O4. RSC Adv. 2017, 7, 47309–47315. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, Q.; Zhu, J.; Luo, L.; Pu, S.; Zhang, W.; Zhu, W.; Sun, J.; Wang, J. Portable Colorimetric Detection of Mercury(II) Based on a Non-Noble Metal Nanozyme with Tunable Activity. Inorg. Chem. 2019, 58, 1638–1646. [Google Scholar] [CrossRef]
- Shah, J.; Singh, S. Unveiling the role of ATP in amplification of intrinsic peroxidase-like activity of gold nanoparticles. 3 Biotech 2018, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Huang, Y.; Ren, J.; Qu, X. Incorporating ATP into biomimetic catalysts for realizing exceptional enzymatic performance over a broad temperature range. NPG Asia Mater. 2014, 6, e114. [Google Scholar] [CrossRef]
- Vallabani, N.V.S.; Karakoti, A.S.; Singh, S. ATP-mediated intrinsic peroxidase-like activity of Fe3O4-based nanozyme: One step detection of blood glucose at physiological pH. Colloids Surf. B 2017, 153, 52–60. [Google Scholar] [CrossRef]
- Singh, M.; Weerathunge, P.; Liyanage, P.D.; Mayes, E.; Ramanathan, R.; Bansal, V. Competitive Inhibition of the Enzyme-Mimic Activity of Gd-Based Nanorods toward Highly Specific Colorimetric Sensing of l-Cysteine. Langmuir 2017, 33, 10006–10015. [Google Scholar] [CrossRef]
- Li, W.; Zhi, X.; Yang, J.; Zhang, J.; Fu, Y. Colorimetric detection of cysteine and homocysteine based on an oligonucleotide-stabilized Pd nanozyme. Anal. Methods 2016, 8, 5111–5116. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, Y.; Nie, G.; Li, M.; Wang, C.; Lu, X. FeCo nanoparticles-embedded carbon nanofibers as robust peroxidase mimics for sensitive colorimetric detection of l-cysteine. Dalton Trans. 2017, 46, 8942–8949. [Google Scholar] [CrossRef]
- Ray, C.; Dutta, S.; Sarkar, S.; Sahoo, R.; Roy, A.; Pal, T. Intrinsic peroxidase-like activity of mesoporous nickel oxide for selective cysteine sensing. J. Mater. Chem. B 2014, 2, 6097–6105. [Google Scholar] [CrossRef]
- Huang, Z.; Yang, Y.; Long, Y.; Zheng, H. A colorimetric method for cysteine determination based on the peroxidase-like activity of ficin. Anal. Methods 2018, 10, 2676–2680. [Google Scholar] [CrossRef]
- Zou, H.; Yang, T.; Lan, J.; Huang, C. Use of the peroxidase mimetic activity of erythrocyte-like Cu1.8S nanoparticles in the colorimetric determination of glutathione. Anal. Methods 2017, 9, 841–846. [Google Scholar] [CrossRef]
- Ganganboina, A.B.; Doong, R.-A. The biomimic oxidase activity of layered V2O5 nanozyme for rapid and sensitive nanomolar detection of glutathione. Sens. Actuators B 2018, 273, 1179–1186. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Lehninger: Principles of Biochemistry. In Priciples of Biochemistry, 7th ed.; Cox, M.M., Ed.; W. H. Freeman and Company: New York, NY, USA, 2017. [Google Scholar]
- Walther, R.; Winther, A.K.; Fruergaard, A.S.; van den Akker, W.; Sørensen, L.; Nielsen, S.M.; Jarlstad Olesen, M.T.; Dai, Y.; Jeppesen, H.S.; Lamagni, P.; et al. Identification and Directed Development of Non-Organic Catalysts with Apparent Pan-Enzymatic Mimicry into Nanozymes for Efficient Prodrug Conversion. Angew. Chem. Int. Ed. 2019, 58, 278–282. [Google Scholar] [CrossRef]
- Kang, T.; Kim, Y.G.; Kim, D.; Hyeon, T. Inorganic nanoparticles with enzyme-mimetic activities for biomedical applications. Coord. Chem. Rev. 2020, 403. [Google Scholar] [CrossRef]
- Chen, W.; Li, S.; Wang, J.; Sun, K.; Si, Y. Metal and metal-oxide nanozymes: Bioenzymatic characteristics, catalytic mechanism, and eco-environmental applications. Nanoscale 2019, 11, 15783–15793. [Google Scholar] [CrossRef]
- Xian, Z.; Zhang, L.; Yu, Y.; Lin, B.; Wang, Y.; Guo, M.; Cao, Y. Nanozyme based on CoFe2O4 modified with MoS2 for colorimetric determination of cysteine and glutathione. Microchim. Acta 2021, 188, 65. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Gupta, V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2. [Google Scholar] [CrossRef]
- Luo, Y.; Duan, G.; Li, G. Synthesis and characterization of flower-like β-Ni(OH)2 nanoarchitectures. J. Solid State Chem. 2007, 180, 2149–2153. [Google Scholar] [CrossRef]
- Kalam, A.; Al-Shihri, A.S.; Al-Sehemi, A.G.; Awwad, N.S.; Du, G.; Ahmad, T. Effect of pH on solvothermal synthesis of β-Ni(OH)2 and NiO nano-architectures: Surface area studies, optical properties and adsorption studies. Superlattice Microst. 2013, 55, 83–97. [Google Scholar] [CrossRef]
- Parveen, N.; Cho, M.H. Self-Assembled 3D Flower-Like Nickel Hydroxide Nanostructures and Their Supercapacitor Applications. Sci. Rep. 2016, 6, 27318. [Google Scholar] [CrossRef] [Green Version]
- Tong, G.-X.; Liu, F.-T.; Wu, W.-H.; Shen, J.-P.; Hu, X.; Liang, Y. Polymorphous α-and β-Ni(OH)2 complex architectures: Morphological and phasal evolution mechanisms and enhanced catalytic activity as non-enzymatic glucose sensors. CrystEngComm 2012, 14, 5963–5973. [Google Scholar] [CrossRef]
- Yang, L.-X.; Zhu, Y.-J.; Tong, H.; Liang, Z.-H.; Wang, W.-W. Hierarchical β-Ni(OH)2 and NiO carnations assembled from nanosheet building blocks. Cryst. Growth Des. 2007, 7, 2716–2719. [Google Scholar] [CrossRef]
- Hall, D.S.; Lockwood, D.J.; Poirier, S.; Bock, C.; MacDougall, B.R. Raman and infrared spectroscopy of alpha and beta phases of thin nickel hydroxide films electrochemically formed on nickel. J. Phys. Chem. A 2012, 116, 6771–6784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taşköprü, T.; Zor, M.; Turan, E. Structural characterization of nickel oxide/hydroxide nanosheets produced by CBD technique. Mater. Res. Bull. 2015, 70, 633–639. [Google Scholar] [CrossRef]
- Mironova-Ulmane, N.; Kuzmin, A.; Steins, I.; Grabis, J.; Sildos, I.; Pärs, M. Raman scattering in nanosized nickel oxide NiO. J. Phys. Conf. Ser. 2007, 93. [Google Scholar] [CrossRef]
- Yu, F.; Huang, Y.; Cole, A.J.; Yang, V.C. The artificial peroxidase activity of magnetic iron oxide nanoparticles and its application to glucose detection. Biomaterials 2009, 30, 4716–4722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weerathunge, P.; Sharma, T.K.; Ramanathan, R.; Bansal, V. CHAPTER 23 Nanozyme-Based Environmental Monitoring. In Advanced Environmental Analysis: Applications of Nanomaterials; The Royal Society of Chemistry: London, UK, 2017; Volume 2, pp. 108–132. [Google Scholar]
- Zhang, X.-Q.; Gong, S.-W.; Zhang, Y.; Yang, T.; Wang, C.-Y.; Gu, N. Prussian blue modified iron oxide magnetic nanoparticles and their high peroxidase-like activity. J. Mater. Chem. 2010, 20, 5110–5116. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, S.; Yin, J.-J.; He, W.; Lu, W.; Ma, M.; Gu, N.; Zhang, Y. Prussian Blue Nanoparticles as Multienzyme Mimetics and Reactive Oxygen Species Scavengers. J. Am. Chem. Soc. 2016, 138, 5860–5865. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, Y.; Wei, H. Nanozymes in bionanotechnology: From sensing to therapeutics and beyond. Inorg. Chem. Front. 2016, 3, 41–60. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Chen, J.; Shu, Y.; Li, H.; Xu, Q.; Hu, X. Nickel metal-organic framework 2D nanosheets with enhanced peroxidase nanozyme activity for colorimetric detection of H2O2. Talanta 2018, 189, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Acker, M.G.; Auld, D.S. Considerations for the design and reporting of enzyme assays in high-throughput screening applications. Perspect. Sci. 2014, 1, 56–73. [Google Scholar] [CrossRef] [Green Version]
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef]
- Lopina, O.D. Enzyme Inhibitors and Activators; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.; Pradhan, M.; Sinha, A.K.; Basu, M.; Negishi, Y.; Pal, T. An Aminolytic Approach toward Hierarchical β-Ni(OH)2 Nanoporous Architectures: A Bimodal Forum for Photocatalytic and Surface-Enhanced Raman Scattering Activity. Inorg. Chem. 2010, 49, 8813–8827. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, N.; Liu, H.; He, Z. Microwave-assisted hydrothermal synthesis of Ni(OH)2 architectures and their in situ thermal convention to NiO. Adv. Powder Technol. 2011, 22, 422–426. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H.; Gong, X.; Li, Q.; Zhao, X. Synthesis, characterization, and cytotoxicity assessment of N-acetyl-l-cysteine capped ZnO nanoparticles as camptothecin delivery system. Colloids Surf. B 2019, 174, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Pawlukojć, A.; Leciejewicz, J.; Ramirez-Cuesta, A.J.; Nowicka-Scheibe, J. L-Cysteine: Neutron spectroscopy, Raman, IR and ab initio study. Spectrochim. Acta A 2005, 61, 2474–2481. [Google Scholar] [CrossRef]
- Strelow, J.; Dewe, W.; Iversen, P.W.; Brooks, H.B.; Radding, J.A.; McGee, J.; Weidner, J. Mechanism of action assays for enzymes. In Assay Guidance Manual; Markossian, S.S.G., Grossman, A., Eds.; Eli Lilly and Company: Indianapolis, IN, USA, 2012. [Google Scholar]
- Luo, D.; Smith, S.W.; Anderson, B.D. Kinetics and Mechanism of the Reaction of Cysteine and Hydrogen Peroxide in Aqueous Solution. J. Pharm. Sci. 2005, 94, 304–316. [Google Scholar] [CrossRef] [PubMed]






| NiO | β-Ni(OH)2 | |||
|---|---|---|---|---|
| ABTS | H2O2 | ABTS | H2O2 | |
| Km (mM) | 17.5 | 14.8 | 7 | 14 |
| Vmax (mM/s) | 5.3 × 10−4 | 1.1 × 10−4 | 2.3 × 10−4 | 8.7 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liyanage, P.D.; Weerathunge, P.; Singh, M.; Bansal, V.; Ramanathan, R. L-Cysteine as an Irreversible Inhibitor of the Peroxidase-Mimic Catalytic Activity of 2-Dimensional Ni-Based Nanozymes. Nanomaterials 2021, 11, 1285. https://doi.org/10.3390/nano11051285
Liyanage PD, Weerathunge P, Singh M, Bansal V, Ramanathan R. L-Cysteine as an Irreversible Inhibitor of the Peroxidase-Mimic Catalytic Activity of 2-Dimensional Ni-Based Nanozymes. Nanomaterials. 2021; 11(5):1285. https://doi.org/10.3390/nano11051285
Chicago/Turabian StyleLiyanage, Piyumi Dinusha, Pabudi Weerathunge, Mandeep Singh, Vipul Bansal, and Rajesh Ramanathan. 2021. "L-Cysteine as an Irreversible Inhibitor of the Peroxidase-Mimic Catalytic Activity of 2-Dimensional Ni-Based Nanozymes" Nanomaterials 11, no. 5: 1285. https://doi.org/10.3390/nano11051285