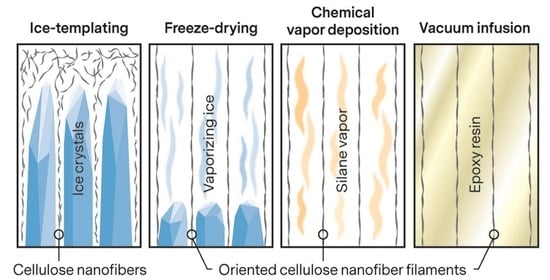
Ice-Templated Cellulose Nanofiber Filaments as a Reinforcement Material in Epoxy Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CNF Filament Preparation
2.3. Preparation of CNF Filament Composites
2.4. Characterization
3. Results and Discussion
3.1. CNF Filament Morphology
3.2. CNF Orientation in the Filaments
3.3. CNF Filament Surface Characteristics
3.4. Composite Morphology
3.5. CNF Orientation in the Composites
3.6. Composite Viscoelastic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, K.-Y.; Aitomäki, Y.; Berglund, L.A.; Oksman, K.; Bismarck, A. On the Use of Nanocellulose as Reinforcement in Polymer Matrix Composites. Compos. Sci. Technol. 2014, 105, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Dufresne, A. Nanocellulose: A New Ageless Bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Yeh, W.Y.; Young, R.J. Molecular Deformation Processes in Aromatic High Modulus Polymer Fibres. Polymer 1999, 40, 857–870. [Google Scholar] [CrossRef]
- Sehaqui, H.; Zhou, Q.; Ikkala, O.; Berglund, L.A. Strong and Tough Cellulose Nanopaper with High Specific Surface Area and Porosity. Biomacromolecules 2011, 12, 3638–3644. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, A. Nanocellulose: From Nature to High Performance Tailored Materials; De Gruyter: Berlin, Germany; Boston, MA, USA, 2012. [Google Scholar] [CrossRef]
- Favier, V.; Canova, G.R.; Cavaillé, J.Y.; Chanzy, H.; Dufresne, A.; Gauthier, C. Nanocomposite Materials from Latex and Cellulose Whiskers. Polym. Adv. Technol. 1995, 6, 351–355. [Google Scholar] [CrossRef]
- Favier, V.; Chanzy, H.; Cavaille, J.Y. Polymer Nanocomposites Reinforced by Cellulose Whiskers. Macromolecules 1995, 28, 6365–6367. [Google Scholar] [CrossRef]
- Nakagaito, A.N.; Yano, H. Novel High-Strength Biocomposites Based on Microfibrillated Cellulose Having Nano-Order-Unit Web-Like Network Structure. Appl. Phys. A 2005, 80, 155–159. [Google Scholar] [CrossRef]
- Henriksson, M.; Fogelström, L.; Berglund, L.A.; Johansson, M.; Hult, A. Novel Nanocomposite Concept Based on Cross-Linking of Hyperbranched Polymers in Reactive Cellulose Nanopaper Templates. Compos. Sci. Technol. 2011, 71, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Aitomäki, Y.; Moreno-Rodriguez, S.; Lundström, T.S.; Oksman, K. Vacuum Infusion of Cellulose Nanofibre Network Composites: Influence of Porosity on Permeability and Impregnation. Mater. Des. 2016, 95, 204–211. [Google Scholar] [CrossRef]
- Hooshmand, S.; Aitomäki, Y.; Norberg, N.; Mathew, A.P.; Oksman, K. Dry-Spun Single-Filament Fibers Comprising Solely Cellulose Nanofibers from Bioresidue. ACS Appl. Mater. Interfaces 2015, 7, 13022–13028. [Google Scholar] [CrossRef]
- Hooshmand, S.; Aitomäki, Y.; Berglund, L.; Mathew, A.P.; Oksman, K. Enhanced Alignment and Mechanical Properties through the Use of Hydroxyethyl Cellulose in Solvent-Free Native Cellulose Spun Filaments. Compos. Sci. Technol. 2017, 150, 79–86. [Google Scholar] [CrossRef]
- Iwamoto, S.; Isogai, A.; Iwata, T. Structure and Mechanical Properties of Wet-Spun Fibers Made from Natural Cellulose Nanofibers. Biomacromolecules 2011, 12, 831–836. [Google Scholar] [CrossRef]
- Walther, A.; Timonen, J.V.I.; Díez, I.; Laukkanen, A.; Ikkala, O. Multifunctional High-Performance Biofibers Based on Wet-Extrusion of Renewable Native Cellulose Nanofibrils. Adv. Mater. 2011, 23, 2924–2928. [Google Scholar] [CrossRef]
- Håkansson, K.M.O.; Fall, A.B.; Lundell, F.; Yu, S.; Krywka, C.; Roth, S.V.; Santoro, G.; Kvick, M.; Prahl Wittberg, L.; Wågberg, L.; et al. Hydrodynamic Alignment and Assembly of Nanofibrils Resulting in Strong Cellulose Filaments. Nat. Commun. 2014, 5, 4018. [Google Scholar] [CrossRef]
- Cunha, A.G.; Lundahl, M.; Ansari, M.F.; Johansson, L.S.; Campbell, J.M.; Rojas, O.J. Surface Structuring and Water Interactions of Nanocellulose Filaments Modified with Organosilanes Toward Wearable Materials. ACS Appl. Nano Mater. 2018, 1, 5279–5288. [Google Scholar] [CrossRef]
- Mittal, N.; Ansari, F.; Gowda, V.K.; Brouzet, C.; Chen, P.; Larsson, P.T.; Roth, S.V.; Lundell, F.; Wågberg, L.; Kotov, N.A.; et al. Multiscale Control of Nanocellulose Assembly: Transferring Remarkable Nanoscale Fibril Mechanics to Macroscale Fibers. ACS Nano 2018, 12, 6378–6388. [Google Scholar] [CrossRef]
- Munier, P.; Gordeyeva, K.; Bergström, L.; Fall, A.B. Directional Freezing of Nanocellulose Dispersions Aligns the Rod-Like Particles and Produces Low-Density and Robust Particle Networks. Biomacromolecules 2016, 17, 1875–1881. [Google Scholar] [CrossRef]
- Pan, Z.Z.; Nishihara, H.; Iwamura, S.; Sekiguchi, T.; Sato, A.; Isogai, A.; Kang, F.; Kyotani, T.; Yang, Q.H. Cellulose Nanofiber as a Distinct Structure-Directing Agent for Xylem-Like Microhoneycomb Monoliths by Unidirectional Freeze-Drying. ACS Nano 2016, 10, 10689–10697. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Wu, T.; Han, D.; Ren, Q.; Siqueira, G.; Nyström, G. Ultralight, Flexible, and Biomimetic Nanocellulose/Silver Nanowire Aerogels for Electromagnetic Interference Shielding. ACS Nano 2020, 14, 2927–2938. [Google Scholar] [CrossRef]
- Barari, B.; Ellingham, T.K.; Qamhia, I.I.; Pillai, K.M.; El-Hajjar, R.; Turng, L.-S.; Sabo, R. Mechanical Characterization of Scalable Cellulose Nano-Fiber Based Composites Made Using Liquid Composite Molding Process. Compos. Part B 2016, 84, 277–284. [Google Scholar] [CrossRef]
- Nissilä, T.; Karhula, S.S.; Saarakkala, S.; Oksman, K. Cellulose Nanofiber Aerogels Impregnated with Bio-Based Epoxy Using Vacuum Infusion: Structure, Orientation and Mechanical Properties. Compos. Sci. Technol. 2018, 155, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Zhai, T.; Zheng, Q.; Cai, Z.; Turng, L.S.; Xia, H.; Gong, S. Poly(Vinyl Alcohol)/Cellulose Nanofibril Hybrid Aerogels with an Aligned Microtubular Porous Structure and Their Composites with Polydimethylsiloxane. Appl. Mater. Interfaces 2015, 7, 7436–7444. [Google Scholar] [CrossRef] [PubMed]
- Kriechbaum, K.; Munier, P.; Apostolopoulou-Kalkavoura, V.; Lavoine, N. Analysis of the Porous Architecture and Properties of Anisotropic Nanocellulose Foams: A Novel Approach to Assess the Quality of Cellulose Nanofibrils (CNFs). ACS Sustain. Chem. Eng. 2018, 6, 11959–11967. [Google Scholar] [CrossRef]
- Chen, W.; Li, Q.; Wang, Y.; Yi, X.; Zeng, J.; Yu, H.; Liu, Y.; Li, J. Comparative Study of Aerogels Obtained from Differently Prepared Nanocellulose Fibers. ChemSusChem 2014, 7, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhou, C.; Wu, Y.; Liu, F.; Wu, Q. Self-Assembling Behavior of Cellulose Nanoparticles during Freeze-Drying: Effect of Suspension Concentration, Particle Size, Crystal Structure, and Surface Charge. Biomacromolecules 2013, 14, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Nissilä, T.; Hietala, M.; Oksman, K. A Method for Preparing Epoxy-Cellulose Nanofiber Composites with an Oriented Structure. Compos. Part A 2019, 125, 105515. [Google Scholar] [CrossRef]
- George, J.; Ivens, J.; Verpoest, I. Mechanical Properties of Flax Fibre Reinforced Epoxy Composites. Angew. Makromol. Chem. 1999, 272, 41–45. [Google Scholar] [CrossRef]
- Abdelmouleh, M.; Boufi, S.; Belgacem, M.N.; Dufresne, A.; Gandini, A. Modification of Cellulose Fibers with Functionalized Silanes: Effect of the Fiber Treatment on the Mechanical Performances of Cellulose-Thermoset Composites. J. Appl. Polym. Sci. 2005, 98, 974–984. [Google Scholar] [CrossRef]
- Hietala, M.; Varrio, K.; Berglund, L.; Soini, J.; Oksman, K. Potential of municipal solid waste paper as raw material for production of cellulose nanofibres. Waste Manag. 2018, 80, 319–326. [Google Scholar] [CrossRef]
- Lundahl, M.J.; Cunha, A.G.; Rojo, E.; Papageorgiou, A.C.; Rautkari, L.; Arboleda, J.C.; Rojas, O.J. Strength and Water Interactions of Cellulose I Filaments Wet-Spun from Cellulose Nanofibril Hydrogels. Sci. Rep. 2016, 6, 30695. [Google Scholar] [CrossRef]
- Sehaqui, H.; Ezekiel Mushi, N.; Morimune, S.; Salajkova, M.; Nishino, T.; Berglund, L.A. Cellulose Nanofiber Orientation in Nanopaper and Nanocomposites by Cold Drawing. ACS Appl. Mater. Interfaces 2012, 4, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Sheng, K.; Yu, K.; Xu, L.; Fontanillo Lopez, C.A. Improved Properties of PLA Biocomposites Toughened with Bamboo Cellulose Nanowhiskers through Silane Modification. J. Mater. Sci. 2018, 53, 10920–10932. [Google Scholar] [CrossRef]
- Fernandes, S.C.M.; Sadocco, P.; Alonso-Varona, A.; Palomares, T.; Eceiza, A.; Silvestre, A.J.D.; Mondragon, I.; Freire, C.S.R. Bioinspired Antimicrobial and Biocompatible Bacterial Cellulose Membranes Obtained by Surface Functionalization with Aminoalkyl Groups. ACS Appl. Mater. Interfaces 2013, 5, 3290–3297. [Google Scholar] [CrossRef]
- Selulosa-Polivinilklorida, S.R.N.; Sheltami, R.M.; Kargarzadeh, H.A.N.I.E.H.; Abdullah, I.B.R.A.H.I.M. Effects of Silane Surface Treatment of Cellulose Nanocrystals on the Tensile Properties of Cellulose-Polyvinyl Chloride Nanocomposite. JSM 2015, 44, 801–810. [Google Scholar] [CrossRef]
- Pacaphol, K.; Aht-Ong, D. The Influences of Silanes on Interfacial Adhesion and Surface Properties of Nanocellulose Film Coating on Glass and Aluminum Substrates. Surf. Coat. Technol. 2017, 320, 70–81. [Google Scholar] [CrossRef]
- Lu, J.; Askeland, P.; Drzal, L.T. Surface Modification of Microfibrillated Cellulose for Epoxy Composite Applications. Polymer 2008, 49, 1285–1296. [Google Scholar] [CrossRef]
- Reale Batista, M.D.; Drzal, L.T. Carbon Fiber/Epoxy Matrix Composite Interphases Modified with Cellulose Nanocrystals. Compos. Sci. Technol. 2018, 164, 274–281. [Google Scholar] [CrossRef]
- Abdelmouleh, M.; Boufi, S.; ben Salah, A.; Belgacem, M.N.; Gandini, A. Interaction of Silane Coupling Agents with Cellulose. Langmuir 2002, 18, 3203–3208. [Google Scholar] [CrossRef]
- Khanjanzadeh, H.; Behrooz, R.; Bahramifar, N.; Gindl-Altmutter, W.; Bacher, M.; Edler, M.; Griesser, T. Surface Chemical Functionalization of Cellulose Nanocrystals by 3-Aminopropyltriethoxysilane. Int. J. Biol. Macromol. 2018, 106, 1288–1296. [Google Scholar] [CrossRef]
- Kontturi, E.J. Surface Chemistry of Cellulose: From Natural Fibres to Model Surfaces. Doctoral thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2005. [Google Scholar]
- Cui, M.; Ren, S.; Qin, S.; Xue, Q.; Zhao, H.; Wang, L. Non-Covalent Functionalized Hexagonal Boron Nitride Nanoplatelets to Improve Corrosion and Wear Resistance of Epoxy Coatings. RSC Adv. 2017, 7, 44043–44053. [Google Scholar] [CrossRef] [Green Version]
- Wada, M.; Sugiyama, J.; Okano, T. Native Celluloses on the Basis of Two Crystalline Phase (Iα/Iβ) System. J. Appl. Polym. Sci. 1993, 49, 1491–1496. [Google Scholar] [CrossRef]
- Wang, B.; Torres-Rendon, J.G.; Yu, J.; Zhang, Y.; Walther, A. Aligned Bioinspired Cellulose Nanocrystal-Based Nanocomposites with Synergetic Mechanical Properties and Improved Hygromechanical Performance. ACS Appl. Mater. Interfaces 2015, 7, 4595–4607. [Google Scholar] [CrossRef]
- Tang, L.; Weder, C. Cellulose Whisker/Epoxy Resin Nanocomposites. ACS Appl. Mater. Interfaces 2010, 2, 1073–1080. [Google Scholar] [CrossRef] [Green Version]








| Sample | O 1s | C 1s | N 1s | Si 2p | (C–C + C–Si): (O–C–O + C–O) |
|---|---|---|---|---|---|
| 1.1 µm silylated | 30.05 | 55.31 | 6.55 | 7.64 | 0.42 |
| 0.56 µm silylated | 40.39 | 50.85 | 3.88 | 4.89 | 0.44 |
| Non-silylated reference | 42.58 | 55.54 | 0.00 | 1.78 | 0.21 |
| Sample | Storage Modulus at 30 °C (MPa) * | Storage Modulus at 100 °C (MPa) * | Tan Delta Peak (°C) * |
|---|---|---|---|
| Epoxy | 2180 (± 180) a | 6.52 (± 0.53) a | 72.1 (± 1.0) a |
| 0.56 µm ‖ | 5030 (± 510) b | 1270 (± 230) b | 71.3 (± 1.1) ab |
| 0.56 µm silylated ‖ | 5050 (± 360) b | 1250 (± 100) b | 80.2 (± 0.4) d |
| 0.56 µm ⊥ | 3270 (± 170) a | 270 (± 64) a | 66.5 (± 0.6) c |
| 0.56 µm silylated ⊥ | 3600 (± 260) a | 381 (± 69) a | 76.4 (± 0.7) e |
| 1.1 µm ‖ | 5350 (± 560) b | 1440 (± 440) b | 73.4 (± 0.5) a |
| 1.1 µm silylated ‖ | 5260 (± 390) b | 1070 (± 170) b | 80.2 (± 0.9) d |
| 1.1 µm ⊥ | 2970 (± 350) a | 187 (± 84) a | 68.8 (± 1.1) bc |
| 1.1 silylated ⊥ | 3360 (± 140) a | 282 (± 91) a | 78.2 (± 0.5) de |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nissilä, T.; Wei, J.; Geng, S.; Teleman, A.; Oksman, K. Ice-Templated Cellulose Nanofiber Filaments as a Reinforcement Material in Epoxy Composites. Nanomaterials 2021, 11, 490. https://doi.org/10.3390/nano11020490
Nissilä T, Wei J, Geng S, Teleman A, Oksman K. Ice-Templated Cellulose Nanofiber Filaments as a Reinforcement Material in Epoxy Composites. Nanomaterials. 2021; 11(2):490. https://doi.org/10.3390/nano11020490
Chicago/Turabian StyleNissilä, Tuukka, Jiayuan Wei, Shiyu Geng, Anita Teleman, and Kristiina Oksman. 2021. "Ice-Templated Cellulose Nanofiber Filaments as a Reinforcement Material in Epoxy Composites" Nanomaterials 11, no. 2: 490. https://doi.org/10.3390/nano11020490