Structural Disorder in Layered Hybrid Halide Perovskites: Types of Stacking Faults, Influence on Optical Properties and Their Suppression by Crystallization Engineering
Abstract
:1. Introduction
2. Materials and Methods
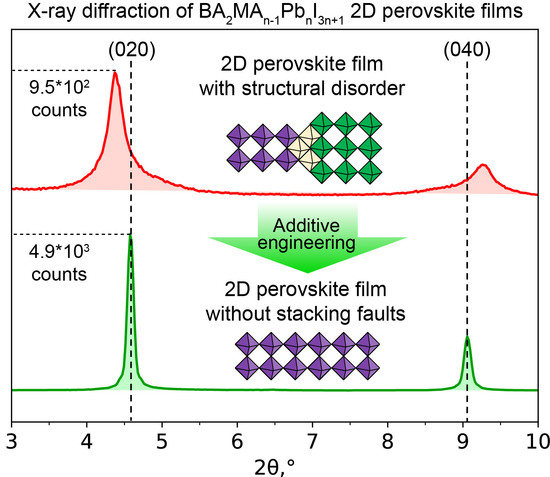
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mao, L.; Stoumpos, C.C.; Kanatzidis, M.G. Two-Dimensional Hybrid Halide Perovskites: Principles and Promises. J. Am. Chem. Soc. 2019, 141, 1171–1190. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Kennard, R.M.; Traore, B.; Ke, W.; Katan, C.; Even, J.; Chabinyc, M.L.; Stoumpos, C.C.; Kanatzidis, M.G. Seven-Layered 2D Hybrid Lead Iodide Perovskites. Chem 2019, 5, 2593–2604. [Google Scholar] [CrossRef]
- Vashishtha, P.; Ng, M.; Shivarudraiah, S.B.; Halpert, J.E. High Efficiency Blue and Green Light-Emitting Diodes Using Ruddlesden-Popper Inorganic Mixed Halide Perovskites with Butylammonium Interlayers. Chem. Mater. 2019, 31, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Udalova, N.; Fateev, S.; Nemygina, E.; Zanetta, A.; Grancini, G.; Goodilin, E.; Tarasov, A. Nonmonotonic photostability of BA2MAn−1PbnI3n+1 homologous layered perovskites. arXiv 2021, arXiv:2109.05989. [Google Scholar]
- Blancon, J.C.; Tsai, H.; Nie, W.; Stoumpos, C.C.; Pedesseau, L.; Katan, C.; Kepenekian, M.; Soe, C.M.M.; Appavoo, K.; Sfeir, M.Y.; et al. Extremely efficient internal exciton dissociation through edge states in layered 2D perovskites. Science 2017, 355, 1288–1292. [Google Scholar] [CrossRef] [Green Version]
- Marchenko, E.I.; Korolev, V.V.; Fateev, S.A.; Mitrofanov, A.; Nickolay, N.; Goodilin, E.A.; Tarasov, A.B. Relationships between distortions of inorganic framework and band gap of layered hybrid halide perovskites. Chem. Mater. 2021, 33, 7518–7526. [Google Scholar] [CrossRef]
- Fu, W.; Wang, J.; Zuo, L.; Gao, K.; Liu, F.; Ginger, D.S.; Jen, A.K.Y. Two-Dimensional Perovskite Solar Cells with 14.1% Power Conversion Efficiency and 0.68% External Radiative Efficiency. ACS Energy Lett. 2018, 3, 2086–2093. [Google Scholar] [CrossRef]
- Tsai, H.; Nie, W.; Blancon, J.-C.; Stoumpos, C.C.; Asadpour, R.; Harutyunyan, B.; Neukirch, A.J.; Verduzco, R.; Crochet, J.J.; Tretiak, S.; et al. High-efficiency two-dimensional Ruddlesden–Popper perovskite solar cells. Nature 2016, 536, 312–316. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, Q.; Chmiel, F.P.; Sakai, N.; Herz, L.M.; Snaith, H.J. Efficient ambient-air-stable solar cells with 2D-3D heterostructured butylammonium-caesium-formamidinium lead halide perovskites. Nat. Energy 2017, 2, 17135. [Google Scholar] [CrossRef]
- Chen, S.; Shen, N.; Zhang, L.; Zhang, L.; Cheung, S.H.; Chen, S. Understanding the Interplay of Binary Organic Spacer in Ruddlesden—Popper Perovskites toward Efficient and Stable Solar Cells. Adv. Funct. Mater. 2020, 30, 1907759. [Google Scholar] [CrossRef]
- Shang, Y.; Liao, Y.; Wei, Q.; Wang, Z.; Xiang, B.; Ke, Y.; Liu, W.; Ning, Z. Highly stable hybrid perovskite light-emitting diodes based on Dion-Jacobson structure. Sci. Adv. 2019, 5, eaaw8072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, M.; Quan, L.N.; Comin, R.; Walters, G.; Sabatini, R.; Voznyy, O.; Hoogland, S.; Zhao, Y.; Beauregard, E.M.; Kanjanaboos, P.; et al. Perovskite energy funnels for efficient light-emitting diodes. Nat. Nanotechnol. 2016, 11, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.; Nie, W.; Blancon, J.C.; Stoumpos, C.C.; Soe, C.M.M.; Yoo, J.; Crochet, J.; Tretiak, S.; Even, J.; Sadhanala, A.; et al. Stable Light-Emitting Diodes Using Phase-Pure Ruddlesden–Popper Layered Perovskites. Adv. Mater. 2018, 30, 1704217. [Google Scholar] [CrossRef]
- Yang, X.; Chu, Z.; Meng, J.; Yin, Z.; Zhang, X.; Deng, J.; You, J. Effects of Organic Cations on the Structure and Performance of Quasi-Two-Dimensional Perovskite-Based Light-Emitting Diodes. J. Phys. Chem. Lett. 2019, 10, 2892–2897. [Google Scholar] [CrossRef]
- Zhang, H.; Liao, Q.; Wu, Y.; Zhang, Z.; Gao, Q.; Liu, P.; Li, M. 2D Ruddlesden—Popper Perovskites Microring Laser Array. Adv. Mater. 2018, 30, 1706186. [Google Scholar] [CrossRef]
- Qin, C.; Sandanayaka, A.S.D.; Zhao, C.; Matsushima, T.; Zhang, D.; Fujihara, T.; Adachi, C. Stable room-temperature continuous-wave lasing in quasi-2D perovskite films. Nature 2020, 585, 53–57. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Zhu, L.; Qin, L.; Wang, Y.; Hu, Y.; Lou, Z.; Hou, Y.; Teng, F. Two-dimensional organic-inorganic hybrid perovskite field-effect transistors with polymers as bottom-gate dielectrics. J. Mater. Chem. C 2019, 7, 4004–4012. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, A.; Noh, Y.Y. Recent progress on metal halide perovskite field-effect transistors. J. Inf. Disp. 2021, 22, 257–268. [Google Scholar] [CrossRef]
- Tan, Z.; Wu, Y.; Hong, H.; Yin, J.; Zhang, J.; Lin, L.; Wang, M.; Sun, X.; Sun, L.; Huang, Y.; et al. Two-Dimensional (C4H9NH3)2 PbBr4 Perovskite Crystals for High-Performance Photodetector. J. Am. Chem. Soc. 2016, 138, 16612–16615. [Google Scholar] [CrossRef]
- Wang, K.; Wu, C.; Yang, D.; Jiang, Y.; Priya, S. Quasi-two-dimensional halide perovskite single crystal photodetector. ACS Nano 2018, 12, 4919–4929. [Google Scholar] [CrossRef]
- Wang, J.; Luo, S.; Lin, Y.; Chen, Y.; Deng, Y.; Li, Z.; Meng, K.; Chen, G.; Huang, T.; Xiao, S.; et al. Templated growth of oriented layered hybrid perovskites on 3D-like perovskites. Nat. Commun. 2020, 11, 582. [Google Scholar] [CrossRef]
- Lin, Y.; Fang, Y.; Zhao, J.; Shao, Y.; Stuard, S.J.; Nahid, M.M.; Ade, H.; Wang, Q.; Shield, J.E.; Zhou, N.; et al. Unveiling the operation mechanism of layered perovskite solar cells. Nat. Commun. 2019, 10, 1008. [Google Scholar] [CrossRef]
- Venkatesan, N.R.; Kennard, R.M.; Decrescent, R.A.; Nakayama, H.; Dahlman, C.J.; Perry, E.E.; Schuller, J.A.; Chabinyc, M.L. Phase Intergrowth and Structural Defects in Organic Metal Halide Ruddlesden-Popper Thin Films. Chem. Mater. 2018, 30, 8615–8623. [Google Scholar] [CrossRef]
- Tan, W.L.; Cheng, Y.-B.; McNeill, C.R. Direct assessment of structural order and evidence for stacking faults in layered hybrid perovskite films from X-ray scattering measurements. J. Mater. Chem. A 2020, 8, 12790–12798. [Google Scholar] [CrossRef]
- MacRae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Cao, D.H.; Clark, D.J.; Young, J.; Rondinelli, J.M.; Jang, J.I.; Hupp, J.T.; Kanatzidis, M.G. Ruddlesden-Popper Hybrid Lead Iodide Perovskite 2D Homologous Semiconductors. Chem. Mater. 2016, 28, 2852–2867. [Google Scholar] [CrossRef]
- Olikhovska, L.; Ustinov, A. Diffraction analysis of perovskite-like oxides containing irregular intergrowths. J. Appl. Crystallogr. 2009, 42, 1–9. [Google Scholar] [CrossRef]
- Marchenko, E.I.; Korolev, V.V.; Mitrofanov, A.; Fateev, S.A.; Goodilin, E.A.; Tarasov, A.B. Layer Shift Factor in Layered Hybrid Perovskites: Univocal Quantitative Descriptor of Composition–Structure–Property Relationships. Chem. Mater. 2021, 33, 1213–1217. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, X.; Wang, M.; Hu, B. Establishing charge-transfer excitons in 2D perovskite heterostructures. Nat. Commun. 2020, 11, 2618. [Google Scholar] [CrossRef]
- Fateev, S.A.; Petrov, A.A.; Marchenko, E.I.; Zubavichus, Y.V.; Khrustalev, V.N.; Petrov, A.V.; Aksenov, S.M.; Goodilin, E.A.; Tarasov, A.B. FA2PbBr4: Synthesis, Structure, and Unusual Optical Properties of Two Polymorphs of Formamidinium-Based Layered (110) Hybrid Perovskite. Chem. Mater. 2021, 33, 1900–1907. [Google Scholar] [CrossRef]
- Fateev, S.A.; Petrov, A.A.; Khrustalev, V.N.; Dorovatovskii, P.V.; Zubavichus, Y.V.; Goodilin, E.A.; Tarasov, A.B. Solution Processing of Methylammonium Lead Iodide Perovskite from γ-Butyrolactone: Crystallization Mediated by Solvation Equilibrium. Chem. Mater. 2018, 30, 5237–5244. [Google Scholar] [CrossRef]
- Tutantsev, A.S.; Udalova, N.N.; Fateev, S.A.; Petrov, A.A.; Chengyuan, W.; Maksimov, E.G.; Goodilin, E.A.; Tarasov, A.B. New Pigeonholing Approach for Selection of Solvents Relevant to Lead Halide Perovskite Processing. J. Phys. Chem. C 2020, 124, 11117–11123. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tutantsev, A.S.; Marchenko, E.I.; Udalova, N.N.; Fateev, S.A.; Goodilin, E.A.; Tarasov, A.B. Structural Disorder in Layered Hybrid Halide Perovskites: Types of Stacking Faults, Influence on Optical Properties and Their Suppression by Crystallization Engineering. Nanomaterials 2021, 11, 3333. https://doi.org/10.3390/nano11123333
Tutantsev AS, Marchenko EI, Udalova NN, Fateev SA, Goodilin EA, Tarasov AB. Structural Disorder in Layered Hybrid Halide Perovskites: Types of Stacking Faults, Influence on Optical Properties and Their Suppression by Crystallization Engineering. Nanomaterials. 2021; 11(12):3333. https://doi.org/10.3390/nano11123333
Chicago/Turabian StyleTutantsev, Andrei S., Ekaterina I. Marchenko, Natalia N. Udalova, Sergey A. Fateev, Eugene A. Goodilin, and Alexey B. Tarasov. 2021. "Structural Disorder in Layered Hybrid Halide Perovskites: Types of Stacking Faults, Influence on Optical Properties and Their Suppression by Crystallization Engineering" Nanomaterials 11, no. 12: 3333. https://doi.org/10.3390/nano11123333