Quantification and Ecological Risk Assessment of Colloidal Fullerenes Nanoparticles in Sediments by Ultrasonic-Assisted Pressurized Liquid Extraction and High Performance Liquid Chromatography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
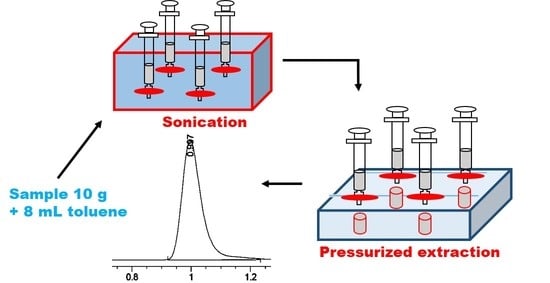
2.2. Apparatus and Glassware
2.3. Instruments
2.4. Sample Collection
2.5. Sample Preparation
2.6. Preparation of Fullerene Solutions
Preparation of Fullerene Working Standard Solution, Spiked Sediment, and Sample Extraction
2.7. Limit of Detection and Limit of Quantification Calculations
2.8. Ecological Risk Calculation
3. Results
3.1. Ultrasonic-Assisted Pressurized Liquid Extraction Method Development
3.1.1. Selection of Extraction Solvent
3.1.2. Selection of Toluene Volume
3.1.3. Evaluation of Sonication Time
3.2. Method Validation
3.2.1. Linearity
3.2.2. Limit of Detection and Limit of Quantification and Recoveries
3.2.3. Precision and Accuracy
4. Discussion
4.1. Environmental Analysis Fullerene
4.2. Ecological Risk Assessment of Fullerenes in Mgeni System Sediments
4.3. Further Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, Z.; Liu, X.; Zhang, W.; Zeng, Z.; Liu, Z.; Zhang, C.; Liu, Y.; Shao, B.; Liang, Q.; Tang, W. Advances in the application, toxicity and degradation of carbon nanomaterials in environment: A review. Environ. Int. 2020, 134, 105298. [Google Scholar] [CrossRef]
- Boxall, A.B.; Tiede, K.; Chaudhry, Q. Engineered nanomaterials in soils and water: How do they behave and could they pose a risk to human health? Nanomedicine 2007, 2, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Tervonen, K.; Waissi, G.; Petersen, E.J.; Akkanen, J.; Kukkonen, J.V.K. Analysis of fullerene-c-60 and kinetic measurements for its accumulation and depuration in daphnia magna. Environ. Toxicol. Chem. 2010, 29, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.M.; Eskridge, K.M.; Li, Y.S. Analysis of the fate and transport of nC(60) nanoparticles in the subsurface using response surface methodology. J. Contam. Hydrol. 2013, 152, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Feng, Z.; Iavicoli, I.; Calabrese, E.J. The two faces of nanomaterials: A quantification of hormesis in algae and plants. Environ. Int. 2019, 131, 105044. [Google Scholar] [CrossRef] [PubMed]
- Nybom, I.; Abel, S.; Waissi, G.; Väänänen, K.; Mäenpää, K.; Leppänen, M.T.; Kukkonen, J.V.; Akkanen, J. Effects of activated carbon on PCB bioaccumulation and biological responses of Chironomus riparius in full life cycle test. Environ. Sci. Technol. 2016, 50, 5252–5260. [Google Scholar] [CrossRef] [PubMed]
- Pakarinen, K.; Petersen, E.; Leppänen, M.; Akkanen, J.; Kukkonen, J. Adverse effects of fullerenes (nC60) spiked to sediments on Lumbriculus variegatus (Oligochaeta). Environ. Pollut. 2011, 159, 3750–3756. [Google Scholar] [CrossRef]
- Pakarinen, K.; Petersen, E.J.; Alvila, L.; Waissi-Leinonen, G.C.; Akkanen, J.; Leppänen, M.T.; Kukkonen, J.V. A screening study on the fate of fullerenes (nC60) and their toxic implications in natural freshwaters. Environ. Toxicol. Chem. 2013, 32, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, J.; Freixa, A.; Lopez-Doval, J.C.; Santos, L.; Sabater, S.; Barcelo, D.; Abad, E.; Farre, M. Bioconcentration and bioaccumulation of C-60 fullerene and C-60 epoxide in biofilms and freshwater snails (Radix sp.). Environ. Res. 2020, 180, 7. [Google Scholar] [CrossRef] [PubMed]
- Ponte, S.; Moore, E.A.; Border, C.T.; Babbitt, C.W.; Tyler, A.C. Fullerene toxicity in the benthos with implications for freshwater ecosystem services. Sci. Total Environ. 2019, 687, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Waissi, G.C.; Vaananen, K.; Nybom, I.; Pakarinen, K.; Akkanen, J.; Leppanen, M.T.; Kukkonen, J.V.K. The chronic effects of fullereneC(60)-associated sediments in the midge Chironomus riparius—Responses in the first and the second generation. Environ. Pollut. 2017, 229, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Parks, A.N.; Chandler, G.T.; Ho, K.T.; Burgess, R.M.; Ferguson, P.L. Environmental biodegradability of [14C] single-walled carbon nanotubes by Trametes versicolor and natural microbial cultures found in New Bedford Harbor sediment and aerated wastewater treatment plant sludge. Environ. Toxicol. Chem. 2015, 34, 247–251. [Google Scholar] [CrossRef]
- Zhang, L.; Petersen, E.J.; Habteselassie, M.Y.; Mao, L.; Huang, Q. Degradation of multiwall carbon nanotubes by bacteria. Environ. Pollut. 2013, 181, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Della Torre, C.; Maggioni, D.; Ghilardi, A.; Parolini, M.; Santo, N.; Landi, C.; Madaschi, L.; Magni, S.; Tasselli, S.; Ascagni, M. The interactions of fullerene C60 and Benzo (α) pyrene influence their bioavailability and toxicity to zebrafish embryos. Environ. Pollut. 2018, 241, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Waissi, G.C.; Bold, S.; Pakarinen, K.; Akkanen, J.; Leppänen, M.T.; Petersen, E.J.; Kukkonen, J.V.K. Chironomus riparius exposure to fullerene-contaminated sediment results in oxidative stress and may impact life cycle parameters. J. Hazard. Mater. 2017, 322, 301–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parolini, M.; Ghilardi, A.; Della Torre, C.; Magni, S.; Prosperi, L.; Calvagno, M.; Del Giacco, L.; Binelli, A. Environmental concentrations of cocaine and its main metabolites modulated antioxidant response and caused cyto-genotoxic effects in zebrafish embryo cells. Environ. Pollut. 2017, 226, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Park, C.M.; Wang, D.J.; Heo, J.; Her, N.; Su, C.M. Aggregation of reduced graphene oxide and its nanohybrids with magnetite and elemental silver under environmentally relevant conditions. J. Nanoparticle Res. 2018, 20, 93. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, C.W.; Usenko, C.Y.; Tanguay, R.L.; Field, J.A. Quantification of fullerenes by LC/ESI-MS and its application to in vivo toxicity assays. Anal. Chem. 2007, 79, 9091–9097. [Google Scholar] [CrossRef]
- Isaacson, C.W.; Kleber, M.; Field, J.A. Quantitative analysis of fullerene nanomaterials in environmental systems: A critical review. Environ. Sci. Technol. 2009, 43, 6463–6474. [Google Scholar] [CrossRef] [PubMed]
- Jehlička, J.; Frank, O.; Hamplová, V.; Pokorná, Z.; Juha, L.; Boháček, Z.; Weishauptová, Z. Low extraction recovery of fullerene from carbonaceous geological materials spiked with C60. Carbon 2005, 43, 1909–1917. [Google Scholar] [CrossRef]
- Jinno, K.; Kohrikawa, C. Supercritical and subcritical fluid extraction of fullerenes from carbon soot. Chim. Oggi 1998, 16, 9–15. [Google Scholar]
- Heymann, D.; Korochantsev, A.; Nazarov, M.; Smit, J. Search for fullerenes C60and C70in Cretaceous–Tertiary boundary sediments from Turkmenistan, Kazakhstan, Georgia, Austria, and Denmark. Cretac. Res. 1996, 17, 367–380. [Google Scholar] [CrossRef]
- Hou, W.C.; Westerhoff, P.; Posner, J.D. Biological accumulation of engineered nanomaterials: A review of current knowledge. Environ. Sci. Process. Impacts 2013, 15, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cai, Q.; Fang, Y.; Anderson, T.A.; Cobb, G.P. Determination of fullerenes (C60) in artificial sediments by liquid chromatography. Talanta 2011, 87, 35–39. [Google Scholar] [CrossRef]
- Sanchís, J.; Aminot, Y.; Abad, E.; Jha, A.N.; Readman, J.W.; Farré, M. Transformation of C60 fullerene aggregates suspended and weathered under realistic environmental conditions. Carbon 2018, 128, 54–62. [Google Scholar] [CrossRef]
- Sanchis, J.; Bosch-Orea, C.; Farre, M.; Barcelo, D. Nanoparticle tracking analysis characterisation and parts-per-quadrillion determination of fullerenes in river samples from Barcelona catchment area. Anal. Bioanal. Chem. 2015, 407, 4261–4275. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, J.; Milacic, R.; Zuliani, T.; Vidmar, J.; Abad, E.; Farre, M.; Barcelo, D. Occurrence of C60 and related fullerenes in the Sava River under different hydrologic conditions. Sci. Total Environ. 2018, 643, 1108–1116. [Google Scholar] [CrossRef]
- Sanchis, J.; Oliveira, L.F.S.; de Leao, F.B.; Farre, M.; Barcelo, D. Liquid chromatography-atmospheric pressure photoionization-Orbitrap analysis of fullerene aggregates on surface soils and river sediments from Santa Catarina (Brazil). Sci. Total Environ. 2015, 505, 172–179. [Google Scholar] [CrossRef]
- Carboni, A.; Emke, E.; Parsons, J.R.; Kalbitz, K.; de Voogt, P. An analytical method for determination of fullerenes and functionalized fullerenes in soils with high performance liquid chromatography and UV detection. Anal. Chim. Acta 2014, 807, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Shareef, A.; Li, G.; Kookana, R.S. Quantitative determination of fullerene (C60) in soils by high performance liquid chromatography and accelerated solvent extraction technique. Environ. Chem. 2010, 7, 292–297. [Google Scholar] [CrossRef]
- Pérez, R.A.; Albero, B.; Miguel, E.; Tadeo, J.L.; Sanchez-Brunete, C. A Rapid Procedure for the Determination of C60 and C70 Fullerenes in Soil and Sediments by Ultrasound-assisted Extraction and HPLC-UV. Anal. Sci. 2013, 29, 533–538. [Google Scholar] [CrossRef] [Green Version]
- Astefanei, A.; Nunez, O.; Galceran, M.T. Analysis of C-60-fullerene derivatives and pristine fullerenes in environmental samples by ultrahigh performance liquid chromatography-atmospheric pressure photoionization-mass spectrometry. J. Chromatogr. A 2014, 1365, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Astefanei, A.; Núñez, O.; Galceran, M.T. Characterisation and determination of fullerenes: A critical review. Anal. Chim. Acta 2015, 882, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Ji, Z.; Guo, W.; Sakkiah, S.; Liu, J.; Patterson, T.A.; Hong, H. Nanomaterial Databases: Data Sources for Promoting Design and Risk Assessment of Nanomaterials. Nanomaterials 2021, 11, 1599. [Google Scholar] [CrossRef]
- Hong, H.; Adam, V.; Nowack, B. Form-Specific and Probabilistic Environmental Risk Assessment of 3 Engineered Nanomaterials (Nano-Ag, Nano-TiO2, and Nano-ZnO) in European Freshwaters. Environ. Toxicol. Chem. 2021, 40, 2629–2639. [Google Scholar] [CrossRef]
- Ahmad, F.; Abubshait, S.A.; Abubshait, H.A. Untargeted metabolomics for Achilles heel of engineered nanomaterials’ risk assessment. Chemosphere 2021, 262. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, S.; Oliveira, J.M.M.; Henriques, I.; Morgado, R.G.; Soares, A.; Loureiro, S. Pollution- induced community tolerance framework-disc diffusion method to assess the impact of silver nanoparticles in soils: Potential relevance for risk assessment. Appl. Soil Ecol. 2022, 169. [Google Scholar] [CrossRef]
- Andreani, T.; Nogueira, V.; Gavina, A.; Fernandes, S.; Rodrigues, J.L.; Pinto, V.V.; Ferreira, M.J.; Silva, A.M.; Pereira, C.M.; Pereira, R. Ecotoxicity to Freshwater Organisms and Cytotoxicity of Nanomaterials: Are We Generating Sufficient Data for Their Risk Assessment? Nanomaterials 2021, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Thomson, M.; Ellison, S.; Wood, R. Harmonized guidelines for single-laboratory validation of methods of analysis. Pure Appl. Chem. 2002, 74, 835. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, P.; Wang, Q.; Huo, X.; Huang, X.; Ye, Q.; Xu, H.; Zhou, G.; Liu, Y.; Zhang, J. Fullerol mediated enhancement of chloramphenicol degradation in Fe (III)/H2O2 system by accelerating Fe (III)/Fe (II) cycle via a non-photochemical pathway. Chem. Eng. J. 2020, 402, 126176. [Google Scholar] [CrossRef]
- Coll, C.; Notter, D.; Gottschalk, F.; Sun, T.; Som, C.; Nowack, B. Probabilistic environmental risk assessment of five nanomaterials (nano-TiO2, nano-Ag, nano-ZnO, CNT, and fullerenes). Nanotoxicology 2016, 10, 436–444. [Google Scholar] [CrossRef] [PubMed]






| Parameters | Validation Results |
|---|---|
| Linearity | 0.01–4 µg g−1 |
| Method detection limit | 0.0094 µg g−1 |
| Method quantification limit | 0.031 µg g−1 |
| Linear regression | 0.9962 |
| Recoveries | 67–84% |
| Precision | ||
| 1 µg g−1 | 4 µg g−1 | |
| Inter-day% RSD | 0.67 | 0.44 |
| Intra-day% RSD | 0.40 | 0.27 |
| Accuracy | ||
| Fortified concentration | 1 µg g−1 | 4 µg g−1 |
| Calculated concentration µg g−1 | 1.08 ± 0.01 | 3.96 ± 0.03 |
| Accuracy (%) | 108 | 99 |
| % Error | 8% | −1% |
| Instrument | Analyte | Extraction Method | Extraction Solvent | Extraction Volume | Sample Mass | Detection Limit | Recovery | Environmental Concentration | Reference |
|---|---|---|---|---|---|---|---|---|---|
| HPLC-UV | C60 & C70 | Ultrasound-assisted extraction | Toluene | 4 mL | 5 g | 0.9 ng g−1 | 72–104% | Not detected | Perez et al. [31] |
| HPLC-UV | C60 | Accelerated solvent extraction | Toluene | 40 mL | 5 g | 20 ng g−1 | 84–107% | - | Shareef et al. [30] |
| HPLC-UV | C60, C70, C61 & C71 | Sonication & shaking extraction | Toluene | 10 mL | 10 g | 3 ng g−1 | 47–71% | - | Carboni et al. [29] |
| HPLC-UV | C60 | Shaking extraction | Toluene | 40 mL | 10 g | 1500 ng g−1 | 83–108% | - | Wang et al. [24] |
| HPLC-UV | C61 | Ultrasonic-assisted pressurized liquid extraction | Toluene | 8 mL | 10 g | 9 ng g−1 | 67–84% | Not detected—30 µg g−1 | Current method |
| Sampling Point | PMB WWTP | Tributary | Before Inanda Dam | After Inanda Dam | DBN WWTP | Estuary |
|---|---|---|---|---|---|---|
| MEC | 23.05 ± 0.12 | 13.27 ± 0.02 | - | 16.87 ± 0.06 | 30.55 ± 0.15 | 10.52 ± 0.10 |
| RCR | 0.92 | 0.53 | - | 0.67 | 1.22 | 0.42 |
| Ecological risk assessment | Medium risk | Medium risk | No risk | Medium risk | High risk | Medium risk |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendricks, N.; Olatunji, O.S.; Gumbi, B.P. Quantification and Ecological Risk Assessment of Colloidal Fullerenes Nanoparticles in Sediments by Ultrasonic-Assisted Pressurized Liquid Extraction and High Performance Liquid Chromatography. Nanomaterials 2021, 11, 3319. https://doi.org/10.3390/nano11123319
Hendricks N, Olatunji OS, Gumbi BP. Quantification and Ecological Risk Assessment of Colloidal Fullerenes Nanoparticles in Sediments by Ultrasonic-Assisted Pressurized Liquid Extraction and High Performance Liquid Chromatography. Nanomaterials. 2021; 11(12):3319. https://doi.org/10.3390/nano11123319
Chicago/Turabian StyleHendricks, Nokwanda, Olatunde Stephen Olatunji, and Bhekumuzi Prince Gumbi. 2021. "Quantification and Ecological Risk Assessment of Colloidal Fullerenes Nanoparticles in Sediments by Ultrasonic-Assisted Pressurized Liquid Extraction and High Performance Liquid Chromatography" Nanomaterials 11, no. 12: 3319. https://doi.org/10.3390/nano11123319