Hierarchical TiO2 Layers Prepared by Plasma Jets
Abstract
:1. Introduction
2. Materials and Methods
2.1. TiO2 Layer Preparation
Spikelet Layers Preparation via Hollow-Cathode Discharge (HCD) Sputtering
P25 Layers Preparation via Spin Coating
2.2. TiO2 Layer Characterization
X-ray Diffraction
Raman Spectroscopy
UV-Vis Spectroscopy
Sorption Experiments
FE-SEM, DF-TEM Coupled with EDAX
TEM
DCDF-TEM
DC Measurements
- (i)
- the total current responses IΣ [A] vs. time t [s] of examined layer to a sequence of irradiation pulses (optical power density 2 mW·cm−2, irradiation duration td = 10 s); the pulses are separated from each other by a dark period of duration td = 10 s and the voltage U applied between measuring points is 500 mV. The responses are presented as IΣ in semilogarithmic scale vs. time t.
- (ii)
- volt-ampere characteristics Id [A] vs. U [V] are measured in the absence of irradiation (dark experiment); the range of applied voltage U was between −500 mV and +500 mV and it was changed in 100-mV increments; these volt-ampere characteristics are straight lines and their slopes Id/U represent the material conductivity σd [S] of the layer.
- (iii)
- volt-ampere characteristics IΣ [A] vs. U [V] are measured upon steady irradiation (optical power density 2 mW·cm−2) using the same voltage sequence as that in item (ii); the slope of the straight lines provides the total conductivity σΣ [S] of the layer.
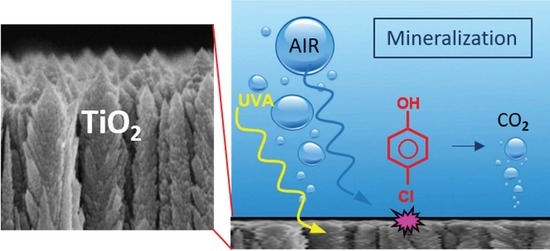
2.3. Photocatalysis in Water and Air
3. Results and Discussion
3.1. Structural Properties of Layers Determined by XRD and Raman Spectroscopy
XRD Spectra
Raman Spectra
UV-Vis Spectra
3.2. Morphology of Layers
Sorption Experiments
SEM
TEM and SAED
DCDF TEM
3.3. DC Measurements
3.4. Photocatalytic Abatement of NOx and Chlorophenol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelly, P.J.; West, G.T.; Ratova, M.; Fisher, L.; Ostovarpour, S.; Verran, J. Structural Formation and Photocatalytic Activity of Magnetron Sputtered Titania and Doped-Titania Coatings. Molecules 2014, 19, 16327–16348. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Rahman, K.H.; Wu, C.-C.; Chen, K.-C. A Review on the Pathways of the Improved Structural Characteristics and Photocatalytic Performance of Titanium Dioxide (TiO2) Thin Layers Fabricated by the Magnetron-Sputtering Technique. Catalysts 2020, 10, 598. [Google Scholar] [CrossRef]
- Navabpour, P.; Ostovarpour, S.; Hampshire, J.; Kelly, P.; Verran, J.; Cooke, K. The effect of process parameters on the structure, photocatalytic and self-cleaning properties of TiO2 and Ag-TiO2 coatings deposited using reactive magnetron sputtering. Thin Solid Layers 2014, 571, 75–83. [Google Scholar] [CrossRef]
- Fresno, F.; González, M.U.; Martínez, L.; Fernández-Castro, M.; Barawi, M.; Villar-García, I.J.; Soler-Morala, J.; Reñones, P.; Luna, M.; Huttel, Y.; et al. Photo-Induced Self-Cleaning and Wettability in TiO2 Nanocolumn Arrays Obtained by Glancing-Angle Deposition with Sputtering. Adv. Sustain. Syst. 2021, 5, 2100071. [Google Scholar] [CrossRef]
- Tuckute, S.; Varnagiris, S.; Urbonavicius, M.; Lelis, M.; Sakalauskaite, S. Tailoring of TiO2 layer crystal texture for higher photocatalysis efficiency. Appl. Surf. Sci. 2019, 489, 576–583. [Google Scholar] [CrossRef]
- Skowronski, L.; Wachowiak, A.A.; Zdunek, K.; Trzcinski, M.; Naparty, M.K. TiO2-based decorative coatings deposited on the AISI 316L stainless steel and glass using an industrial scale magnetron. Thin Solid Layers 2017, 627, 1–8. [Google Scholar] [CrossRef]
- Di Fonzo, F.; Casari, C.S.; Russo, V.; Brunella, M.F.; Li Bassi, A.; Bottani, C.E. Hierarchically organized nanostructured TiO2 for photocatalysis applications. Nanotechnology 2009, 20, 015604. [Google Scholar] [CrossRef] [Green Version]
- Ishii, K.; Kurokawa, K.; Yoshihara, S. High-Rate Deposition of Titanium Dioxide Layers with Photocatalytic Activities by Gas Flow Sputtering. IEICE Trans. Electron. 2004, 87, 232–237. [Google Scholar]
- Kment, S.; Kluson, P.; Hubicka, Z.; Krysa, J.; Cada, M.; Gregora, I.; Deyneka, A.; Remes, Z.; Zabova, H.; Jastrabik, L. Double hollow cathode plasma jet-low temperature method for the TiO2−xNx photoresponding layers. Electrochim. Acta 2010, 55, 1548–1556. [Google Scholar] [CrossRef]
- Kubo, Y.; Iwabuchi, Y.; Yoshikawa, M.; Sato, Y.; Shigesato, Y. High rate deposition of photocatalytic TiO2 layers with high activity by hollow cathode gas-flow sputtering method. J. Vac. Sci. Technol. A 2008, 26, 893–897. [Google Scholar] [CrossRef]
- Sakuma, H.; Ishii, K. Gas flow sputtering: Versatile process for the growth of nanopillars, nanoparticles, and epitaxial thin layers. J. Magn. Magn. Mater. 2009, 321, 872–875. [Google Scholar] [CrossRef]
- Ekeroth, S.; Ikeda, S.; Boyd, R.D.; Shimizu, T.; Helmersson, U. Growth of semi-coherent Ni and NiO dual-phase nanoparticles using hollow cathode sputtering. J. Nanoparticle Res. 2019, 21, 37. [Google Scholar] [CrossRef] [Green Version]
- Gunnarsson, R.; Brenning, N.; Boyd, R.D.; Helmersson, U. Nucleation of titanium nanoparticles in an oxygen-starved environment. I: Experiments. J. Phys. D Appl. Phys. 2018, 51, 455201. [Google Scholar] [CrossRef] [Green Version]
- Gunnarsson, R.; Pilch, I.; Boyd, R.D.; Brenning, N.; Helmersson, U. The influence of pressure and gas flow on size and morphology of titanium oxide nanoparticles synthesized by hollow cathode sputtering. J. Appl. Phys. 2016, 120, 044308. [Google Scholar] [CrossRef] [Green Version]
- Olejníček, J.; Šmíd, J.; Čada, M.; Kšírová, P.; Kohout, M.; Perekrestov, R.; Tvarog, D.; Kment, Š.; Kmentová, H.; Hubička, Z. High rate deposition of photoactive TiO2 layers by hot hollow cathode. Surf. Coat. Technol. 2020, 383, 125256. [Google Scholar] [CrossRef]
- Olejníček, J.; Šmíd, J.; Perekrestov, R.; Kšírová, P.; Rathouský, J.; Kohout, M.; Dvořáková, M.; Kment, Š.; Jurek, K.; Čada, M.; et al. Co3O4 thin layers prepared by hollow cathode discharge. Surf. Coat. Technol. 2019, 366, 303–310. [Google Scholar] [CrossRef]
- Olejníček, J.; Hrubantová, A.; Volfová, L.; Dvořáková, M.; Kohout, M.; Tvarog, D.; Gedeon, O.; Wulff, H.; Hippler, R.; Hubička, Z. WO3 and WO3-x thin films prepared by DC hollow cathode discharge. Vacuum 2021, 110679. [Google Scholar] [CrossRef]
- Hubička, Z.; Pribil, G.; Soukup, R.J.; Ianno, N.J. Investigation of the rf and dc hollow cathode plasma-jet sputtering systems for the deposition of silicon thin layers. Surf. Coat. Technol. 2002, 160, 114–123. [Google Scholar] [CrossRef]
- Mikuška, P.; Motyka, K.; Večeřa, Z. Determination of nitrous acid in air using wet effluent diffusion denuder–FIA technique. Talanta 2008, 77, 635–641. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the enhanced photocatalytic activity of Degussa P25 mixed-phase TiO2 using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Bickley, R.I.; Gonzalez-Carreno, T.; Lees, J.S.; Palmisano, L.; Tilley, R.J.D. A structural investigation of titanium dioxide photocatalysts. J. Solid State Chem. 1991, 92, 178–190. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef]
- Zouzelka, R.; Rathousky, J. Photocatalytic abatement of NOx pollutants in the air using commercial functional coating with porous morphology. Appl. Catal. B Environ. 2017, 217, 466–476. [Google Scholar] [CrossRef]
- Guerin, V.M.; Zouzelka, R.; Bibova-Lipsova, H.; Jirkovsky, J.; Rathousky, J.; Pauporte, T. Experimental and DFT study of the degradation of 4-chlorophenol on hierarchical micro-/nanostructured oxide films. Appl. Catal. B Environ. 2015, 168, 132–140. [Google Scholar] [CrossRef]
- Zouzelka, R.; Kusumawati, Y.; Remzova, M.; Rathousky, J.; Pauporté, T. Photocatalytic activity of porous multiwalled carbon nanotube-TiO2 composite layers for pollutant degradation. J. Hazard. Mater. 2016, 317, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Zouzelka, R.; Remzova, M.; Plsek, J.; Brabec, L.; Rathousky, J. Immobilized rGO/TiO2 photocatalyst for decontamination of water. Catalysts 2019, 9, 708. [Google Scholar] [CrossRef] [Green Version]
- Bredemeyer, N.; De Buhr, S.; Hesse, D. Parameters influencing photoassisted adsorption of NO on TiO2 powder. Chem. Eng. Technol. 2000, 23, 527–533. [Google Scholar] [CrossRef]







| λ/nm | E/eV | Ph/s−1 | σPh/S | σd /S, σΣ/S | Id, IΣ/A | IPh/A | e−/s−1 | EQE |
|---|---|---|---|---|---|---|---|---|
| Dark | - | - | 0 | 2.6E−11 | 1.3E−11 | - | - | - |
| 465 | 2.66 | 4.7E+16 | 1.4E−11 | 4.1E−11 | 2.0E−11 | 0.7E−11 | 4.6E+07 | 9.8E−10 |
| 405 | 3.06 | 2.0E+16 | 4.2E−09 | 4.5E−09 | 2.2E−09 | 2.2E−09 | 1.4E+10 | 6.8E−07 |
| 370 | 3.30 | 4.7E+15 | 3.3E−09 | 3.3E−09 | 1.7E−09 | 1.6E−09 | 1.0E+10 | 2.2E−06 |
| λ/nm | E/eV | Ph/s−1 | σPh/S | σd/S, σΣ/S | Id, IΣ/A | IPh/A | e−/s−1 | EQE |
|---|---|---|---|---|---|---|---|---|
| Dark | - | - | 0 | 4.3E−10 | 2.1E−10 | - | - | - |
| 465 | 2.66 | 4.7E+16 | 0.6E−11 | 4.3E−10 | 2.2E−10 | 3.0E−11 | 1.9E+08 | 4.0E−09 |
| 405 | 3.06 | 2.0E+16 | 0.8E−11 | 5.0E−10 | 2.5E−10 | 3.8E−11 | 2.4E+08 | 1.2E−08 |
| 370 | 3.30 | 4.7E+15 | 0.9E−11 | 5.1E−10 | 2.6E−10 | 4.3E−11 | 2.7E+08 | 5.3E−08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zouzelka, R.; Olejnicek, J.; Ksirova, P.; Hubicka, Z.; Duchon, J.; Martiniakova, I.; Muzikova, B.; Mergl, M.; Kalbac, M.; Brabec, L.; et al. Hierarchical TiO2 Layers Prepared by Plasma Jets. Nanomaterials 2021, 11, 3254. https://doi.org/10.3390/nano11123254
Zouzelka R, Olejnicek J, Ksirova P, Hubicka Z, Duchon J, Martiniakova I, Muzikova B, Mergl M, Kalbac M, Brabec L, et al. Hierarchical TiO2 Layers Prepared by Plasma Jets. Nanomaterials. 2021; 11(12):3254. https://doi.org/10.3390/nano11123254
Chicago/Turabian StyleZouzelka, Radek, Jiri Olejnicek, Petra Ksirova, Zdenek Hubicka, Jan Duchon, Ivana Martiniakova, Barbora Muzikova, Martin Mergl, Martin Kalbac, Libor Brabec, and et al. 2021. "Hierarchical TiO2 Layers Prepared by Plasma Jets" Nanomaterials 11, no. 12: 3254. https://doi.org/10.3390/nano11123254