Boron Modified Bifunctional Cu/SiO2 Catalysts with Enhanced Metal Dispersion and Surface Acid Sites for Selective Hydrogenation of Dimethyl Oxalate to Ethylene Glycol and Ethanol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Catalytic Reaction
3. Results and Discussion
3.1. Structural and Textural Properties of the xB-Cu/SiO2 Catalysts
3.2. XRD and TEM
3.3. H2-TPR and NH3-TPD
3.4. XPS and XAES
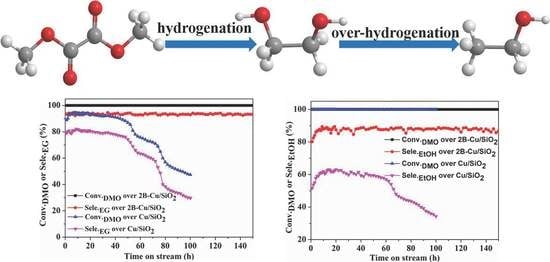
3.5. Catalytic Performance Test
3.6. Structure–Performance Relationship
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, H.H.; Tan, J.J.; Zhu, Y.L.; Zheng, H.Y.; Li, Y.W. Efficient hydrogenation of dimethyl oxalate to methyl glycolate over highly active immobilized-ruthenium catalyst. J. Mol. Catal. A Chem. 2016, 425, 68–75. [Google Scholar] [CrossRef]
- Sheng, H.B.; Zhang, H.T.; Ma, H.F.; Qian, W.X.; Ying, W.Y. An effective Cu-Ag/HMS bimetallic catalyst for hydrogenation of methyl acetate to ethanol. Catal. Today 2020, 358, 122–128. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, W.L.; Yao, D.W.; Wang, S.P.; Xu, Y.; Zhao, Y.J.; Ma, X.B. Effect of surface hydroxyl group of ultra-small silica on the chemical states of copper catalyst for dimethyl oxalate hydrogenation. Catal. Today 2020, 350, 127–135. [Google Scholar] [CrossRef]
- Wang, Z.B.; Ban, L.J.; Meng, P.F.; Li, H.T.; Zhao, Y.X. Ethynylation of Formaldehyde over CuO/SiO2 Catalysts Modified by Mg Species: Effects of the Existential States of Mg Species. Nanomaterials 2019, 9, 1137. [Google Scholar] [CrossRef] [Green Version]
- Ye, R.P.; Lin, L.; Wang, L.C.; Ding, D.; Zhou, Z.F.; Pan, P.B.; Xu, Z.H.; Liu, J.; Adidharma, H.; Radosz, M.; et al. Perspectives on the active sites and catalyst design for the hydrogenation of dimethyl oxalate. ACS Catal. 2020, 10, 4465–4490. [Google Scholar] [CrossRef]
- Chen, C.C.; Lin, L.; Ye, R.P.; Huang, L.; Zhu, L.B.; Huang, Y.Y.; Qin, Y.Y.; Yao, Y.G. Construction of Cu-Ce composite oxides by simultaneous ammonia evaporation method to enhance catalytic performance of Ce-Cu/SiO2 catalysts for dimethyl oxalate hydrogenation. Fuel 2021, 290, 120083. [Google Scholar] [CrossRef]
- Ye, C.L.; Guo, C.L.; Sun, C.W.; Zhang, Y. Effect of Mn doping on the activity and stability of Cu-SiO2 catalysts for the hydrogenation of methyl acetate to ethanol. RSC Adv. 2016, 6, 113796–113802. [Google Scholar] [CrossRef]
- Yin, A.Y.; Wen, C.; Guo, X.Y.; Dai, W.L.; Fan, K.N. Influence of Ni species on the structural evolution of Cu/SiO2 catalyst for the chemoselective hydrogenation of dimethyl oxalate. J. Catal. 2011, 280, 77–88. [Google Scholar] [CrossRef]
- Yin, A.Y.; Qu, J.W.; Guo, X.Y.; Dai, W.L.; Fan, K.N. The influence of B-doping on the catalytic performance of Cu/HMS catalyst for the hydrogenation of dimethyl oxalate. Appl. Catal. A Gen. 2011, 400, 39–47. [Google Scholar] [CrossRef]
- Yin, A.Y.; Guo, X.Y.; Dai, W.L.; Fan, K.N. The nature of active copper species in Cu-HMS catalyst for hydrogenation of dimethyl oxalate to ethylene glycol: New insights on the synergetic effect between Cu0 and Cu+. J. Phys. Chem. C 2009, 113, 11003–11013. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, C.L.; Guo, C.L.; Gan, C.N.; Tong, X.M. In2O3-modified Cu/SiO2 as an active and stable catalyst for the hydrogenation of methyl acetate to ethanol. Chin. J. Catal. 2018, 39, 99–108. [Google Scholar] [CrossRef]
- Yin, A.Y.; Guo, X.Y.; Dai, W.L.; Li, H.X.; Fan, K.N. Highly active and selective copper-containing HMS catalyst in the hydrogenation of dimethyl oxalate to ethylene glycol. Appl. Catal. A Gen. 2008, 349, 91–99. [Google Scholar] [CrossRef]
- Lin, H.; Zheng, X.; He, Z.; Zheng, J.; Duan, X.; Yuan, Y. Cu/SiO2 hybrid catalysts containing HZSM-5 with enhanced activity and stability for selective hydrogenation of dimethyl oxalate to ethylene glycol. Appl. Catal. A Gen. 2012, 445, 287–296. [Google Scholar] [CrossRef]
- He, Z.; Lin, H.Q.; He, P.; Yuan, Y.Z. Effect of boric oxide doping on the stability and activity of a Cu–SiO2 catalyst for vapor-phase hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2011, 277, 54–63. [Google Scholar] [CrossRef]
- Yin, A.Y.; Guo, X.Y.; Fan, K.N.; Dai, W.L. Ion-exchange temperature effect on Cu/HMS Catalysts for the hydrogenation of dimethyl oxalate to ethylene glycol. ChemCatChem 2010, 2, 206–213. [Google Scholar] [CrossRef]
- Chen, L.F.; Guo, P.J.; Qiao, M.H.; Yan, S.R.; Li, H.X.; Shen, W.; Xu, H.L.; Fan, K.N. Cu/SiO2 catalysts prepared by the ammonia-evaporation method: Texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2008, 257, 172–180. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Xu, Z.N.; Peng, S.Y.; Zhang, M.J.; Lu, G.; Chen, Q.S.; Chen, Y.M.; Guo, G.C. High-performance and long-lived Cu/SiO2 nanocatalyst for CO2 hydrogenation. ACS Catal. 2015, 5, 4255–4259. [Google Scholar] [CrossRef]
- Zheng, X.L.; Lin, H.Q.; Zheng, J.W.; Duan, X.P.; Yuan, Y.Z. Lanthanum oxide-modified Cu/SiO2 as a high-performance catalyst for chemoselective hydrogenation of dimethyl oxalate to ethylene glycol. ACS Catal. 2013, 3, 2738–2749. [Google Scholar] [CrossRef]
- Zheng, J.W.; Lin, H.Q.; Wang, Y.N.; Zheng, X.L.; Duan, X.P.; Yuan, Y.Z. Efficient low-temperature selective hydrogenation of esters on bimetallic Au–Ag/SBA-15 catalyst. J. Catal. 2013, 297, 110–118. [Google Scholar] [CrossRef]
- Huang, Y.; Ariga, H.; Zheng, X.L.; Duan, X.P.; Takakusagi, S.; Asakura, K.; Yuan, Y.Z. Silver-modulated SiO2-supported copper catalysts for selective hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2013, 307, 74–83. [Google Scholar] [CrossRef]
- Wang, Q.; Qiu, L.; Ding, D.; Chen, Y.Z.; Shi, C.W.; Cui, P.; Wang, Y.; Zhang, Q.H.; Liu, R.; Shen, H. Performance enhancement of Cu/SiO2 catalyst for hydrogenation of dimethyl oxalate to ethylene glycol through zinc incorporation. Catal. Commun. 2018, 108, 68–72. [Google Scholar] [CrossRef]
- Ye, R.P.; Lin, L.; Liu, C.Q.; Chen, C.C.; Yao, Y.G. One-pot synthesis of cyclodextrin-doped Cu-SiO2 catalysts for efficient hydrogenation of dimethyl oxalate to ethylene glycol. ChemCatChem 2017, 9, 4587–4597. [Google Scholar] [CrossRef]
- Chen, C.C.; Lin, L.; Ye, R.P.; Sun, M.L.; Yang, J.X.; Li, F.; Yao, Y.G. Mannitol as a novel dopant for Cu/SiO2: A low-cost, environmental and highly stable catalyst for dimethyl oxalate hydrogenation without hydrogen prereduction. J. Catal. 2020, 389, 421–431. [Google Scholar] [CrossRef]
- Ren, Z.H.; Younis, M.N.; Li, C.S.; Li, Z.X.; Yang, X.G.; Wang, G.Y. Highly active Ce, Y, La-modified Cu/SiO2 catalysts for hydrogenation of methyl acetate to ethanol. RSC Adv. 2020, 10, 5590–5603. [Google Scholar] [CrossRef]
- Zhu, S.H.; Gao, X.Q.; Zhu, Y.L.; Zhu, Y.F.; Zheng, H.Y.; Li, Y.W. Promoting effect of boron oxide on Cu/SiO2 catalyst for glycerol hydrogenolysis to 1,2-propanediol. J. Catal. 2013, 303, 70–79. [Google Scholar] [CrossRef]
- Zhao, S.; Yue, H.R.; Zhao, Y.J.; Wang, B.; Geng, Y.C.; Lv, J.; Wang, S.P.; Gong, J.L.; Ma, X.B. Chemoselective synthesis of ethanol via hydrogenation of dimethyl oxalate on Cu/SiO2: Enhanced stability with boron dopant. J. Catal. 2013, 297, 142–150. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalytic properties of ceria and CeO2-containing materials. Catal. Rev. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- Dandekar, A.; Vannice, M.A. Determination of the dispersion and surface oxidation states of supported Cu catalysts. J. Catal. 1998, 178, 621–639. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Li, S.M.; Wang, Y.; Shan, B.; Zhang, J.; Wang, S.P.; Ma, X.B. Efficient tuning of surface copper species of Cu/SiO2 catalyst for hydrogenation of dimethyl oxalate to ethylene glycol. Chem. Eng. J. 2017, 313, 759–768. [Google Scholar] [CrossRef]
- Mao, D.S.; Chen, Q.L.; Lu, G.Z. Vapor-phase Beckmann rearrangement of cyclohexanone oxime over B2O3/TiO2-ZrO2. Appl. Catal. A Gen. 2003, 244, 273–282. [Google Scholar] [CrossRef]
- Song, Y.B.; Zhang, J.; Lv, J.; Zhao, Y.J.; Ma, X.B. Hydrogenation of dimethyl oxalate over copper-based catalysts: Acid–base properties and reaction paths. Ind. Eng. Chem. Res. 2015, 54, 9699–9707. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Xu, Z.N.; Zhang, M.J.; Chen, Q.S.; Chen, Y.M.; Guo, G.C. Insight into composition evolution in the synthesis of high-performance Cu/SiO2 catalysts for CO2 hydrogenation. RSC Adv. 2016, 6, 25185–25190. [Google Scholar] [CrossRef]
- Sun, K.P.; Lu, W.W.; Qiu, F.Y.; Liu, S.W.; Xu, X.L. Direct synthesis of DME over bifunctional catalyst: Surface properties and catalytic performance. Appl. Catal. A Gen. 2003, 252, 243–249. [Google Scholar] [CrossRef]
- Van Der Grift, C.J.G.; Elberse, P.A.; Mulder, A.; Geus, J.W. Preparation of silica-supported copper catalysts by means of deposition-precipitation. Appl. Catal. 1990, 59, 275–289. [Google Scholar] [CrossRef]
- Van Der Grift, C.J.G.; Wielers, A.F.H.; Jogh, B.P.J.; Van Beunum, J.; De Boer, M.; Versluijs-Helder, M.; Geus, J.W. Effect of the reduction treatment on the structure and reactivity of silica-supported copper particles. J. Catal. 1991, 131, 178–189. [Google Scholar] [CrossRef]
- Busca, G. FT-IR study of the surface of copper oxide. J. Mol. Catal. 1987, 43, 225–236. [Google Scholar] [CrossRef]
- van Engelen, M.C.; Teunissen, H.T.; de Vries, J.G.; Elsevier, C.J. Suitable ligands for homogeneous ruthenium-catalyzed hydrogenolysis of esters. J. Mol. Catal. A Chem. 2003, 206, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Carlini, C.; Di Girolamo, M.; Macinai, A.; Marchionna, M.; Noviello, M.; Maria Raspolli Galletti, A.; Sbrana, G. Selective synthesis of isobutanol by means of the Guerbet reaction: Part 2. Reaction of methanol/ethanol and methanol/ethanol/n-propanol mixtures over copper based/MeONa catalytic systems. J. Mol. Catal. A Chem. 2003, 200, 137–146. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, S.; Geng, Y.; Shen, Y.; Yue, H.; Lv, J.; Wang, S.; Ma, X. Ni-containing Cu/SiO2 catalyst for the chemoselective synthesis of ethanol via hydrogenation of dimethyl oxalate. Catal. Today 2016, 276, 28–35. [Google Scholar] [CrossRef]
- Ye, R.P.; Lin, L.; Yang, J.X.; Sun, M.L.; Li, F.; Li, B.; Yao, Y.G. A new low-cost and effective method for enhancing the catalytic performance of Cu–SiO2 catalysts for the synthesis of ethylene glycol via the vapor-phase hydrogenation of dimethyl oxalate by coating the catalysts with dextrin. J. Catal. 2017, 350, 122–132. [Google Scholar] [CrossRef]
- Gong, J.L.; Yue, H.L.; Zhao, Y.J.; Zhao, S.; Zhao, L.; Lv, J.; Wang, S.P.; Ma, X.B. Synthesis of ethanol via syngas on Cu/SiO2 catalysts with balanced Cu0-Cu+ sites. J. Am. Chem. Soc. 2012, 134, 13922–13925. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.L.; Ma, X.B.; Gong, J.L. An alternative synthetic approach for efficient catalytic conversion of syngas to ethanol. Acc. Chem. Res. 2014, 47, 1483–1492. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Zhao, Y.; Lv, J.; Wang, S.; Ma, X. Insight into the balancing effect of active Cu species for hydrogenation of carbon–oxygen bonds. ACS Catal. 2015, 5, 6200–6208. [Google Scholar] [CrossRef]
- Gong, W.; Chen, C.; Zhang, Y.; Zhou, H.; Wang, H.; Zhang, H.; Zhang, Y.; Wang, G.; Zhao, H. Efficient synthesis of furfuryl alcohol from H2-hydrogenation/transfer hydrogenation of furfural using sulfonate group modified Cu catalyst. ACS Sustain. Chem. Eng. 2017, 5, 2172–2180. [Google Scholar] [CrossRef]
- Huang, X.M.; Ma, M.; Miao, S.; Zheng, Y.P.; Chen, M.S.; Shen, W.J. Hydrogenation of methyl acetate to ethanol over a highly stable Cu/SiO2 catalyst: Reaction mechanism and structural evolution. Appl. Catal. A Gen. 2017, 531, 79–88. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Zhang, H.H.; Xu, Y.X.; Wang, S.N.; Xu, Y.; Wang, S.P.; Ma, X.B. Interface tuning of Cu+/Cu0 by zirconia for dimethyl oxalate hydrogenation to ethylene glycol over Cu/SiO2 catalyst. J. Energy Chem. 2020, 49, 248–256. [Google Scholar] [CrossRef]












| Catalysts | Cu Loading (wt%) a | B Loading (wt%) a | Cu Dispersion (%) b | SCu (m2·g−1) b | SBET (m2·g−1) | VP (cm3·g−1) c | Dp (nm) d |
|---|---|---|---|---|---|---|---|
| Cu/SiO2 | 14.34 | 0 | 17.9 | 19.2 | 386.0 | 0.42 | 3.8 |
| 0.25B-Cu/SiO2 | 14.23 | 0.60 | 18.5 | 19.6 | 402.5 | 0.42 | 3.9 |
| 1B-Cu/SiO2 | 14.06 | 2.38 | 19.9 | 21.1 | 421.7 | 0.45 | 4.1 |
| 2B-Cu/SiO2 | 13.94 | 4.72 | 21.6 | 23.8 | 449.3 | 0.48 | 4.2 |
| 3B-Cu/SiO2 | 13.88 | 7.11 | 20.3 | 21.7 | 427.1 | 0.44 | 4.0 |
| Catalyst | Total NH3 Desorbed (μmol/g) | Density of Acidic Sites (μmol/m2) |
|---|---|---|
| Cu/SiO2 catalyst | 76 | 0.20 |
| 0.25B-Cu/SiO2 catalyst | 89 | 0.22 |
| 1B-Cu/SiO2 catalyst | 102 | 0.24 |
| 2B-Cu/SiO2 catalyst | 126 | 0.28 |
| 3B-Cu/SiO2 catalyst | 137 | 0.32 |
| Catalyst | K.E. (eV) a | A.P. (eV) b | Cu2p3/2 B.E. (eV) | XCu+ (%) c | ||
|---|---|---|---|---|---|---|
| Cu+ | Cu0 | Cu+ | Cu0 | |||
| Cu/SiO2 | 913.8 | 918.3 | 1846.3 | 1850.8 | 932.5 | 59.5 |
| 0.25B-Cu/SiO2 | 913.8 | 918.4 | 1846.4 | 1851.0 | 932.6 | 60.4 |
| 1B-Cu/SiO2 | 914.2 | 918.5 | 1846.7 | 1851.0 | 932.5 | 62.7 |
| 2B-Cu/SiO2 | 914.2 | 918.5 | 1846.8 | 1851.1 | 932.6 | 64.6 |
| 3B-Cu/SiO2 | 914.4 | 918.6 | 1846.8 | 1851.2 | 932.6 | 69.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Ye, R.; Lin, L.; Guo, R.; Zhao, P.; Yin, Y.; Cheng, W.; Yuan, W.; Yao, Y. Boron Modified Bifunctional Cu/SiO2 Catalysts with Enhanced Metal Dispersion and Surface Acid Sites for Selective Hydrogenation of Dimethyl Oxalate to Ethylene Glycol and Ethanol. Nanomaterials 2021, 11, 3236. https://doi.org/10.3390/nano11123236
Yang D, Ye R, Lin L, Guo R, Zhao P, Yin Y, Cheng W, Yuan W, Yao Y. Boron Modified Bifunctional Cu/SiO2 Catalysts with Enhanced Metal Dispersion and Surface Acid Sites for Selective Hydrogenation of Dimethyl Oxalate to Ethylene Glycol and Ethanol. Nanomaterials. 2021; 11(12):3236. https://doi.org/10.3390/nano11123236
Chicago/Turabian StyleYang, Deliang, Runping Ye, Ling Lin, Rong Guo, Peiyu Zhao, Yanchao Yin, Wei Cheng, Wenpeng Yuan, and Yuangen Yao. 2021. "Boron Modified Bifunctional Cu/SiO2 Catalysts with Enhanced Metal Dispersion and Surface Acid Sites for Selective Hydrogenation of Dimethyl Oxalate to Ethylene Glycol and Ethanol" Nanomaterials 11, no. 12: 3236. https://doi.org/10.3390/nano11123236