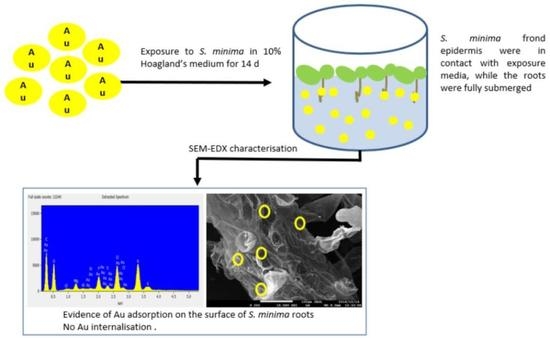
Interactions of Coated-Gold Engineered Nanoparticles with Aquatic Higher Plant Salvinia minima Baker
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of nAu
2.2. Preparation of Exposure Medium and Concentrations
2.3. Test Organism Maintenance
2.4. Biomass Determination
2.5. Interactions of nAu with S. minima
2.5.1. Determination of Total Au Concentrations
2.5.2. Internalization/Uptake of nAu by S. minima
2.5.3. Adsorption of nAu by S. minima
2.6. Data Analysis
3. Results and Discussion
3.1. Characterization of nAu
3.2. Fresh Biomass
3.3. Total Au Analysis in Plant Tissues
3.4. Mechanism of nAu Accumulation by S. minima
4. Environmental Implications
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, A.; Ng, H.P.; Xu, Y.; Li, Y.; Zheng, Y.; Yu, J.; Han, F.; Peng, F.; Fu, L. Gold Nanoparticles: Synthesis, Stability Test, and Application for the Rice Growth. J. Nanomater. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Abadeer, N.S.; Murphy, C.J. Recent Progress in Cancer Thermal Therapy Using Gold Nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, G.; You, M.; Song, E.; Shukoor, M.I.; Zhang, K.; Altman, M.B.; Chen, Y.; Zhu, Z.; Huang, C.Z.; et al. Assembly of Aptamer Switch Probes and Photosensitizer on Gold Nanorods for Targeted Photothermal and Photodynamic Cancer Therapy. ACS Nano 2012, 6, 5070–5077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priecel, P.; Adekunle Salami, H.; Padilla, R.H.; Zhong, Z.; Lopez-Sanchez, J.A. Anisotropic Gold Nanoparticles: Preparation and Applications in Catalysis. Chin. J. Catal. 2016, 37, 1619–1650. [Google Scholar] [CrossRef] [Green Version]
- Bodelón, G.; Costas, C.; Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M. Gold Nanoparticles for Regulation of Cell Function and Behavior. Nano Today 2017, 13, 40–60. [Google Scholar] [CrossRef]
- Musee, N. Simulated Environmental Risk Estimation of Engineered Nanomaterials: A Case of Cosmetics in Johannesburg City. Hum. Exp. Toxicol. 2011, 30, 1181–1195. [Google Scholar] [CrossRef]
- Giese, B.; Klaessig, F.; Park, B.; Kaegi, R.; Steinfeldt, M.; Wigger, H.; von Gleich, A.; Gottschalk, F. Risks, Release and Concentrations of Engineered Nanomaterial in the Environment. Sci. Rep. 2018, 8, 1565. [Google Scholar] [CrossRef]
- Thwala, M.; Klaine, S.J.; Musee, N. Interactions of Metal-Based Engineered Nanoparticles with Aquatic Higher Plants: A Review of the State of Current Knowledge: Engineered Nanoparticle Interactions with Aquatic Plants. Environ. Toxicol. Chem. 2016, 35, 1677–1694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahaye, N.; Thwala, M.; Cowan, D.A.; Musee, N. Genotoxicity of Metal Based Engineered Nanoparticles in Aquatic Organisms: A Review. Mutat. Res. Mutat. Res. 2017, 773, 134–160. [Google Scholar] [CrossRef]
- Ma, X.; Geiser-Lee, J.; Deng, Y.; Kolmakov, A. Interactions between Engineered Nanoparticles (ENPs) and Plants: Phytotoxicity, Uptake and Accumulation. Sci. Total Environ. 2010, 408, 3053–3061. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Das, A.K.; Patel, M.K.; Shah, A.; Kumar, V.; Gantait, S. Engineered Nanomaterials for Plant Growth and Development: A Perspective Analysis. Sci. Total Environ. 2018, 630, 1413–1435. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. Impacts of Silver Nanoparticles on Plants: A Focus on the Phytotoxicity and Underlying Mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef]
- Brunner, T.J.; Wick, P.; Manser, P.; Spohn, P.; Grass, R.N.; Limbach, L.K.; Bruinink, A.; Stark, W.J. In Vitro Cytotoxicity of Oxide Nanoparticles: Comparison to Asbestos, Silica, and the Effect of Particle Solubility. Environ. Sci. Technol. 2006, 40, 4374–4381. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.-J.; Wang, H.; Yan, B.; Zheng, H.; Jiang, Y.; Miranda, O.R.; Rotello, V.M.; Xing, B.; Vachet, R.W. Effect of Surface Charge on the Uptake and Distribution of Gold Nanoparticles in Four Plant Species. Environ. Sci. Technol. 2012, 46, 12391–12398. [Google Scholar] [CrossRef]
- Bäuerlein, P.S.; Emke, E.; Tromp, P.; Hofman, J.A.M.H.; Carboni, A.; Schooneman, F.; de Voogt, P.; van Wezel, A.P. Is There Evidence for Man-Made Nanoparticles in the Dutch Environment? Sci. Total Environ. 2017, 576, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Iswarya, V.; Bhuvaneshwari, M.; Chandrasekaran, N.; Mukherjee, A. Trophic Transfer Potential of Two Different Crystalline Phases of TiO2 NPs from Chlorella Sp. to Ceriodaphnia Dubia. Aquat. Toxicol. 2018, 197, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Thwala, M.; Musee, N.; Sikhwivhilu, L.; Wepener, V. The Oxidative Toxicity of Ag and ZnO Nanoparticles towards the Aquatic Plant Spirodela Punctuta and the Role of Testing Media Parameters. Environ. Sci. Process. Impacts 2013, 15, 1830. [Google Scholar] [CrossRef] [PubMed]
- Song, U.; Lee, S. Phytotoxicity and Accumulation of Zinc Oxide Nanoparticles on the Aquatic Plants Hydrilla Verticillata and Phragmites Australis: Leaf-Type-Dependent Responses. Environ. Sci. Pollut. Res. 2016, 23, 8539–8545. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Bielmyer-Fraser, G.K. Accumulation and Effects of Dissolved and Nanoparticle Silver and Copper in Two Marine Seaweed Species. Ga. J. Sci. 2019, 77, 1. [Google Scholar]
- Glenn, J.B.; White, S.A.; Klaine, S.J. Interactions of Gold Nanoparticles with Freshwater Aquatic Macrophytes Are Size and Species Dependent: Interactions of AuNPs with Freshwater Aquatic Plants. Environ. Toxicol. Chem. 2012, 31, 194–201. [Google Scholar] [CrossRef]
- Raliya, R.; Franke, C.; Chavalmane, S.; Nair, R.; Reed, N.; Biswas, P. Quantitative Understanding of Nanoparticle Uptake in Watermelon Plants. Front. Plant Sci. 2016, 7, 1288. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.F.; Rylott, E.L.; Anderson, C.W.N.; Bruce, N.C. Investigating the Toxicity, Uptake, Nanoparticle Formation and Genetic Response of Plants to Gold. PLoS ONE 2014, 9, e93793. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Bai, X.; Ye, Z.; Ma, L.; Liang, L. Toxicological Responses of Fe3O4 Nanoparticles on Eichhornia Crassipes and Associated Plant Transportation. Sci. Total Environ. 2019, 671, 558–567. [Google Scholar] [CrossRef]
- Judy, J.D.; Unrine, J.M.; Bertsch, P.M. Evidence for Biomagnification of Gold Nanoparticles within a Terrestrial Food Chain. Environ. Sci. Technol. 2011, 45, 776–781. [Google Scholar] [CrossRef]
- Judy, J.D.; Unrine, J.M.; Rao, W.; Wirick, S.; Bertsch, P.M. Bioavailability of Gold Nanomaterials to Plants: Importance of Particle Size and Surface Coating. Environ. Sci. Technol. 2012, 46, 8467–8474. [Google Scholar] [CrossRef]
- Bleeker, E.A.J.; de Jong, W.H.; Geertsma, R.E.; Groenewold, M.; Heugens, E.H.W.; Koers-Jacquemijns, M.; van de Meent, D.; Popma, J.R.; Rietveld, A.G.; Wijnhoven, S.W.P.; et al. Considerations on the EU Definition of a Nanomaterial: Science to Support Policy Making. Regul. Toxicol. Pharmacol. 2013, 65, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Khataee, A.; Movafeghi, A.; Mojaver, N.; Vafaei, F.; Tarrahi, R.; Dadpour, M.R. Toxicity of Copper Oxide Nanoparticles on Spirodela Polyrrhiza: Assessing Physiological Parameters. Res. Chem. Intermed. 2017, 43, 927–941. [Google Scholar] [CrossRef]
- Varga, M.; Horvatić, J.; Barišić, L.; Lončarić, Z.; Dutour Sikirić, M.; Erceg, I.; Kočić, A.; Štolfa Čamagajevac, I. Physiological and Biochemical Effect of Silver on the Aquatic Plant Lemna Gibba L.: Evaluation of Commercially Available Product Containing Colloidal Silver. Aquat. Toxicol. 2019, 207, 52–62. [Google Scholar] [CrossRef]
- Regier, N.; Cosio, C.; von Moos, N.; Slaveykova, V.I. Effects of Copper-Oxide Nanoparticles, Dissolved Copper and Ultraviolet Radiation on Copper Bioaccumulation, Photosynthesis and Oxidative Stress in the Aquatic Macrophyte Elodea Nuttallii. Chemosphere 2015, 128, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Milewska-Hendel, A.; Zubko, M.; Karcz, J.; Stróż, D.; Kurczyńska, E. Fate of Neutral-Charged Gold Nanoparticles in the Roots of the Hordeum Vulgare L. Cultivar Karat. Sci. Rep. 2017, 7, 3014. [Google Scholar] [CrossRef] [Green Version]
- Baalousha, M.; Yang, Y.; Vance, M.E.; Colman, B.P.; McNeal, S.; Xu, J.; Blaszczak, J.; Steele, M.; Bernhardt, E.; Hochella, M.F. Outdoor Urban Nanomaterials: The Emergence of a New, Integrated, and Critical Field of Study. Sci. Total Environ. 2016, 557–558, 740–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prado, C.; Rodríguez-Montelongo, L.; González, J.A.; Pagano, E.A.; Hilal, M.; Prado, F.E. Uptake of Chromium by Salvinia minima: Effect on Plant Growth, Leaf Respiration and Carbohydrate Metabolism. J. Hazard. Mater. 2010, 177, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Mahaye, N. Stability of Gold and Cerium Oxide Nanoparticles in Aqueous Environments, and Their Effects on Pseudokirchneriella Subcapitata and Salvinia minima. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2019. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Hu, C.; Liu, Y.; Li, X.; Li, M. Biochemical Responses of Duckweed (Spirodela Polyrhiza) to Zinc Oxide Nanoparticles. Arch. Environ. Contam. Toxicol. 2013, 64, 643–651. [Google Scholar] [CrossRef]
- Auffan, M.; Rose, J.; Wiesner, M.R.; Bottero, J.-Y. Chemical Stability of Metallic Nanoparticles: A Parameter Controlling Their Potential Cellular Toxicity In Vitro. Environ. Pollut. 2009, 157, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Iswarya, V.; Manivannan, J.; De, A.; Paul, S.; Roy, R.; Johnson, J.B.; Kundu, R.; Chandrasekaran, N.; Mukherjee, A.; Mukherjee, A. Surface Capping and Size-Dependent Toxicity of Gold Nanoparticles on Different Trophic Levels. Environ. Sci. Pollut. Res. 2016, 23, 4844–4858. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Jin, X.; George, S.; Xia, T.; Meng, H.; Wang, X.; Suarez, E.; Zhang, H.; Hoek, E.M.V.; Godwin, H.; et al. Dispersion and Stability Optimization of TiO2 Nanoparticles in Cell Culture Media. Environ. Sci. Technol. 2010, 44, 7309–7314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahaye, N.; Leareng, S.K.; Musee, N. Cytotoxicity and Genotoxicity of Coated-Gold Nanoparticles on Freshwater Algae Pseudokirchneriella Subcapitata. Aquat. Toxicol. 2021, 236, 105865. [Google Scholar] [CrossRef]
- Booth, A.; Størseth, T.; Altin, D.; Fornara, A.; Ahniyaz, A.; Jungnickel, H.; Laux, P.; Luch, A.; Sørensen, L. Freshwater Dispersion Stability of PAA-Stabilised Cerium Oxide Nanoparticles and Toxicity towards Pseudokirchneriella Subcapitata. Sci. Total Environ. 2015, 505, 596–605. [Google Scholar] [CrossRef] [Green Version]
- Römer, I.; White, T.A.; Baalousha, M.; Chipman, K.; Viant, M.R.; Lead, J.R. Aggregation and Dispersion of Silver Nanoparticles in Exposure Media for Aquatic Toxicity Tests. J. Chromatogr. A 2011, 1218, 4226–4233. [Google Scholar] [CrossRef]
- Marimuthu, K.; Subramaniam, R.; Lertanantawong, B.; Lee, S.; Borgio, J.; Amin, S.; Azeez, S.A.; Rahman, M.; Arshad, A. Toxicity of Gold Nanoparticles on the Survival and Hatching Rates of African Catfish (Clarias Gariepinus) Embryo and Larvae. J. Environ. Biol. 2020, 41, 1179–1185. [Google Scholar] [CrossRef]
- Barreto, Â.; Luis, L.G.; Girão, A.V.; Trindade, T.; Soares, A.M.V.M.; Oliveira, M. Behavior of Colloidal Gold Nanoparticles in Different Ionic Strength Media. J. Nanoparticle Res. 2015, 17, 493. [Google Scholar] [CrossRef]
- Hitchman, A.; Sambrook Smith, G.H.; Ju-Nam, Y.; Sterling, M.; Lead, J.R. The Effect of Environmentally Relevant Conditions on PVP Stabilised Gold Nanoparticles. Chemosphere 2013, 90, 410–416. [Google Scholar] [CrossRef]
- Diegoli, S.; Manciulea, A.L.; Begum, S.; Jones, I.P.; Lead, J.R.; Preece, J.A. Interaction between Manufactured Gold Nanoparticles and Naturally Occurring Organic Macromolecules. Sci. Total Environ. 2008, 402, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Feichtmeier, N.S.; Walther, P.; Leopold, K. Uptake, Effects, and Regeneration of Barley Plants Exposed to Gold Nanoparticles. Environ. Sci. Pollut. Res. 2015, 22, 8549–8558. [Google Scholar] [CrossRef]
- Pinto, R.J.B.; Marques, P.A.A.P.; Martins, M.A.; Neto, C.P.; Trindade, T. Electrostatic Assembly and Growth of Gold Nanoparticles in Cellulosic Fibres. J. Colloid Interface Sci. 2007, 312, 506–512. [Google Scholar] [CrossRef]
- Pereira, S.O.; Barros-Timmons, A.; Trindade, T. Biofunctionalisation of Colloidal Gold Nanoparticles via Polyelectrolytes Assemblies. Colloid Polym. Sci. 2014, 292, 33–50. [Google Scholar] [CrossRef]
- Barrena, R.; Casals, E.; Colón, J.; Font, X.; Sánchez, A.; Puntes, V. Evaluation of the Ecotoxicity of Model Nanoparticles. Chemosphere 2009, 75, 850–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostroumov, S.A.; Poklonov, V.A.; Kotelevtsev, S.V.; Orlov, S.N. Toxicity of Gold Nanoparticles for Plants in Experimental Aquatic System. Mosc. Univ. Biol. Sci. Bull. 2014, 69, 108–112. [Google Scholar] [CrossRef] [Green Version]
- Palácio, S.M.; Nogueira, D.A.; Espinoza-Quiñones, F.R.; de Campos, É.A.; Veit, M.T. Silver Nanoparticles Bioaccumulation by Aquatic Macrophyte Salvinia Auriculata. Water Air Soil Pollut. 2020, 231, 62. [Google Scholar] [CrossRef]
- Nelson, B.C.; Petersen, E.J.; Marquis, B.J.; Atha, D.H.; Elliott, J.T.; Cleveland, D.; Watson, S.S.; Tseng, I.-H.; Dillon, A.; Theodore, M.; et al. NIST Gold Nanoparticle Reference Materials Do Not Induce Oxidative DNA Damage. Nanotoxicology 2013, 7, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Goswami, S. Copper Phytoextraction by Salvinia Cucullata: Biochemical and Morphological Study. Environ. Sci. Pollut. Res. 2017, 24, 1363–1371. [Google Scholar] [CrossRef]
- Shi, J.; Peng, C.; Yang, Y.; Yang, J.; Zhang, H.; Yuan, X.; Chen, Y.; Hu, T. Phytotoxicity and Accumulation of Copper Oxide Nanoparticles to the Cu-Tolerant Plant Elsholtzia Splendens. Nanotoxicology 2014, 8, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.R.; Beaulieu, A.L.; Beaulieu, N.L.; Mazer, S.J.; Keller, A.A. Environmental Stresses IncreasePhotosynthetic Disruption by Metal Oxide Nanomaterials in a Soil-Grown Plant. ACS Nano 2015, 9, 11737–11749. [Google Scholar] [CrossRef] [Green Version]
- Thwala, M.; Klaine, S.; Musee, N. Exposure Media and Nanoparticle Size Influence on the Fate, Bioaccumulation, and Toxicity of Silver Nanoparticles to Higher Plant Salvinia minima. Molecules 2021, 26, 2305. [Google Scholar] [CrossRef]
- Denny, P. Sites of Nutrient Absorption in Aquatic Macrophytes. J. Ecol. 1972, 60, 819–829. [Google Scholar] [CrossRef]
- Lin, S.; Reppert, J.; Hu, Q.; Hudson, J.S.; Reid, M.L.; Ratnikova, T.A.; Rao, A.M.; Luo, H.; Ke, P.C. Uptake, Translocation, and Transmission of Carbon Nanomaterials in Rice Plants. Small 2009, 5, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Milewska-Hendel, A.; Zubko, M.; Stróż, D.; Kurczyńska, E.U. Effect of Nanoparticles Surface Charge on the Arabidopsis thaliana (L.) Roots Development and Their Movement into the Root Cells and Protoplasts. Int. J. Mol. Sci. 2019, 20, 1650. [Google Scholar] [CrossRef] [Green Version]
- Castro-Longoria, E.; Trejo-Guillén, K.; Vilchis-Nestor, A.R.; Avalos-Borja, M.; Andrade-Canto, S.B.; Leal-Alvarado, D.A.; Santamaría, J.M. Biosynthesis of Lead Nanoparticles by the Aquatic Water Fern, Salvinia minima Baker, When Exposed to High Lead Concentration. Colloids Surf. B Biointerfaces 2014, 114, 277–283. [Google Scholar] [CrossRef]
- Stegemeier, J.P.; Colman, B.P.; Schwab, F.; Wiesner, M.R.; Lowry, G.V. Uptake and Distribution of Silver in the Aquatic Plant Landoltia Punctata (Duckweed) Exposed to Silver and Silver Sulfide Nanoparticles. Environ. Sci. Technol. 2017, 51, 4936–4943. [Google Scholar] [CrossRef] [PubMed]
- Judy, J.D.; McNear, D.H.; Chen, C.; Lewis, R.W.; Tsyusko, O.V.; Bertsch, P.M.; Rao, W.; Stegemeier, J.; Lowry, G.V.; McGrath, S.P.; et al. Nanomaterials in Biosolids Inhibit Nodulation, Shift Microbial Community Composition, and Result in Increased Metal Uptake Relative to Bulk/Dissolved Metals. Environ. Sci. Technol. 2015, 49, 8751–8758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hua, T.; Xiao, F.; Chen, C.; Gersberg, R.M.; Liu, Y.; Ng, W.J.; Tan, S.K. Uptake and Accumulation of CuO Nanoparticles and CdS/ZnS Quantum Dot Nanoparticles by Schoenoplectus Tabernaemontani in Hydroponic Mesocosms. Ecol. Eng. 2014, 70, 114–123. [Google Scholar] [CrossRef]
- Li, L.; Sillanpää, M.; Tuominen, M.; Lounatmaa, K.; Schultz, E. Behavior of Titanium Dioxide Nanoparticles in Lemna Minor Growth Test Conditions. Ecotoxicol. Environ. Saf. 2013, 88, 89–94. [Google Scholar] [CrossRef]
- Glenn, J.B.; Klaine, S.J. Abiotic and Biotic Factors That Influence the Bioavailability of Gold Nanoparticles to Aquatic Macrophytes. Environ. Sci. Technol. 2013, 47, 10223–10230. [Google Scholar] [CrossRef]
- Zhang, D.; Hua, T.; Xiao, F.; Chen, C.; Gersberg, R.M.; Liu, Y.; Stuckey, D.; Ng, W.J.; Tan, S.K. Phytotoxicity and Bioaccumulation of ZnO Nanoparticles in Schoenoplectus Tabernaemontani. Chemosphere 2015, 120, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Movafeghi, A.; Khataee, A.; Abedi, M.; Tarrahi, R.; Dadpour, M.; Vafaei, F. Effects of TiO2 Nanoparticles on the Aquatic Plant Spirodela Polyrrhiza: Evaluation of Growth Parameters, Pigment Contents and Antioxidant Enzyme Activities. J. Environ. Sci. 2018, 64, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Liu, X.; Li, X.; Zhao, Y. Evaluation of Growth and Biochemical Indicators of Salvinia Natans Exposed to Zinc Oxide Nanoparticles and Zinc Accumulation in Plants. Environ. Sci. Pollut. Res. 2014, 21, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Adani, F.; Papa, G.; Schievano, A.; Cardinale, G.; D’Imporzano, G.; Tambone, F. Nanoscale Structure of the Cell Wall Protecting Cellulose from Enzyme Attack. Environ. Sci. Technol. 2011, 45, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Lizieri, C.; Aguiar, R.; Kuki, K.N. Manganese Accumulation and Its Effects on Three Tropical Aquatic Macrophytes: Azolla caroliniana, Salvinia mínima and Spirodela polyrhiza. Rodriguésia 2011, 62, 909–917. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, I.I.; Espadas-Gil, F.; Talavera-May, C.; Fuentes, G.; Santamaría, J.M. Capacity of the Aquatic Fern (Salvinia minima Baker) to Accumulate High Concentrations of Nickel in Its Tissues, and Its Effect on Plant Physiological Processes. Aquat. Toxicol. 2014, 155, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Leal-Alvarado, D.A.; Espadas-Gil, F.; Sáenz-Carbonell, L.; Talavera-May, C.; Santamaría, J.M. Lead Accumulation Reduces Photosynthesis in the Lead Hyper-Accumulator Salvinia minima Baker by Affecting the Cell Membrane and Inducing Stomatal Closure. Aquat. Toxicol. 2016, 171, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Lahive, E.; O’Halloran, J.; Jansen, M.A.K. A Marriage of Convenience; a Simple Food Chain Comprised of Lemna minor (L.) and Gammarus pulex (L.) to Study the Dietary Transfer of Zinc. Plant Biol. 2015, 17, 75–81. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H.; Zhang, Y.; Xin, H. The Effect of CuO NPs on Reactive Oxygen Species and Cell Cycle Gene Expression in Roots of Rice. Environ. Toxicol. Chem. 2015, 34, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Asli, S.; Neumann, P.M. Colloidal Suspensions of Clay or Titanium Dioxide Nanoparticles Can Inhibit Leaf Growth and Transpiration via Physical Effects on Root Water Transport. Plant Cell Environ. 2009, 32, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fernández, D.; Barroso, D.; Komárek, M. Root Water Transport of Helianthus Annuus L. under Iron Oxide Nanoparticle Exposure. Environ. Sci. Pollut. Res. 2016, 23, 1732–1741. [Google Scholar] [CrossRef] [PubMed]





| Plant | Mechanism | Detection Method | ENP Type | ENP Properties | Exposure Media | Duration | Dosage | Controlling Factor | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Azolla caroliniana | Internalization | TEM, STEM, SEM, EDX | Au | 4 nm; 18 nm; spherical −14.1 mV; ζ −9.73 mV | Borehole water; pH 7.1; TOC;8.56 mg/L; CaCO3 107 mg/conductivity 210 mS/cm | 24 h | 250 µg/L | Species type: internalization due to the presence of root hairs used by the plant to acquire nutrients | [20] |
| Egeria densa | Adsorption | TEM, STEM, SEM, EDX | Au | 4 nm; 18 nm; spherical; ζ −14.1 mV; ζ −9.73 mV | Borehole water; pH 7.1; TOC;8.56 mg/L; CaCO3 107 mg/conductivity 210 mS/cm | 24 h | 250 µg/L | Presence of root hairs facilitated internalization | [20] |
| Lemna minor | Adsorption (cell wall of leaves) | SEM; TEM | TiO2 | 275–2398 nm; SSA 50 m2/g; | Steinburg growth medium, pH 5.5; CaCO3 166 mg/L | 14 d | 0.01–10 mg/L | Exposure concentration: Accumulation increased with an increase exposure concentration | [64] |
| Myriophyllum simulans | Adsorption | TEM, STEM, SEM, EDX | Au | 4 nm; spherical; ζ −14.1 mV | Borehole water; pH 7.1; TOC;8.56 mg/L; CaCO3 107 mg/conductivity 210 mS/cm | 24 h | 250 µg/L | Size: High accumulation from 4 nm Au NPs. | [20] |
| Salvinia auriculata | Absorption | ICP-MS | Ag | 100 nm, PVP-coated | Cultivation media with 14/10 h (light/dark) cycle and temperature between 23 and 24 °C in a greenhouse | 64 d | 1–10 mg/L | Absorption increased with exposure time, and nAg concentration | [51] |
| Salvinia minima | Adsorption (cell wall of leaves) | TEM, SEM, XPS | Pb | spherical, 17.2 ± 4.2 nm | Hoagland’s medium | 12 h | 80 mg/L | Morphology: Spherical NPs were found within the cell wall while elongated ones were associated with the cell membrane. | [60] |
| Salvinia minima | Adsorption (cell wall of roots) | TEM, SEM, XPS | Pb | Elongated, 53.7 ± 29.6 nm in length and 11.1 ± 2.4 nm wide | Hoagland’s medium | 12 h | 80 mg/L | Spherical shaped NPs were within the cell wall while elongated ones were associated with the cell membrane | [60] |
| Salvinia minima | Adsorption (roots and leaves) | TEM, SEM, ICP-MS | Au | 5, 20, 40 nm; spherical; citrate and BPEI coated | 10% Hoagland’s medium; pH 7 | 14 d | 1 mg/L | Exposure media: high agglomeration of NPs leading to lack of internalization | [current study] |
| Salvinia natans | Adsorption | ICP-OES | ZnO | 25 nm; uncoated; SSA; 90 m2/g; 1–10 mg/L | OECD growth medium; pH 6.5 | 7 d | 1–50 mg/L | Concentration: High agglomeration and settling of NPs at 20 and 50 mg/L | [68] |
| Schoenoplectus tabernaemontani | Internalization (roots) | TEM | CuO; CdS QDs | 38 nm; SSA 12.84 m2/g; ζ −2.8 mV | Hoagland’s medium | 21 d | 0.5–50 mg/L | NP type: Root uptake percentage for nCuO treatment ranged from 40.6 to 68.4%, while the values were 8.7 to 21.3% for CdS QDs | [63] |
| Schoenoplectus tabernaemontani | Internalization | SEM; TEM | ZnO | 35 nm; SSA 43 m2/g; ζ −5.4 mV (start), −2.6 mV (end) | Nutrient solution, pH 6.4–6.8 | 21 d | 10–1000 mg/L | Particulate vs. ionic form: Uptake of Zn from nZnO was greater than that for Zn2+. | [66] |
| Spirodela polyrrhiza | Internalization | Epifluorescence microscopy | TiO2 | 8 nm, anatase | 50% S. polyrrhiza specific culture medium | 6 d | 0.05–10 mg/L | Structural characteristics: Anatase and crystalline nTiO2 allow their remarkable movement into the root cells | [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahaye, N.; Thwala, M.; Musee, N. Interactions of Coated-Gold Engineered Nanoparticles with Aquatic Higher Plant Salvinia minima Baker. Nanomaterials 2021, 11, 3178. https://doi.org/10.3390/nano11123178
Mahaye N, Thwala M, Musee N. Interactions of Coated-Gold Engineered Nanoparticles with Aquatic Higher Plant Salvinia minima Baker. Nanomaterials. 2021; 11(12):3178. https://doi.org/10.3390/nano11123178
Chicago/Turabian StyleMahaye, Ntombikayise, Melusi Thwala, and Ndeke Musee. 2021. "Interactions of Coated-Gold Engineered Nanoparticles with Aquatic Higher Plant Salvinia minima Baker" Nanomaterials 11, no. 12: 3178. https://doi.org/10.3390/nano11123178