Facile Synthesis of Microporous Carbons from Biomass Waste as High Performance Supports for Dehydrogenation of Formic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Catalysts
2.3. Test of Catalysts
2.4. Calculation Method
2.5. Characterization of Catalysts
3. Results and Discussion
3.1. Catalyst Characterization Results
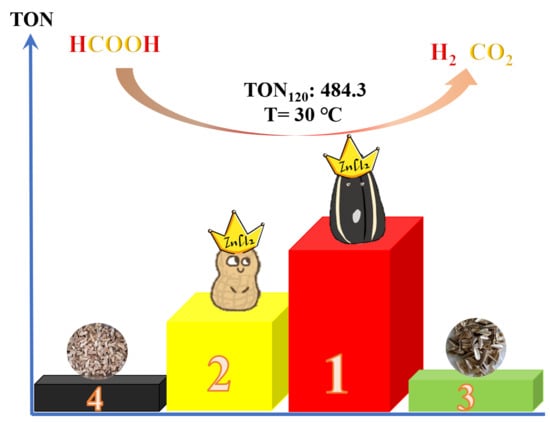
3.2. Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, Y.; Xue, Y.; Liu, T.; Liu, Y.; Zhang, C.; Xing, C.; He, F.; Li, Y. Bimetallic mixed clusters highly loaded on porous 2D graphdiyne for hydrogen energy conversion. Adv. Sci. 2021, 8, 2102777. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Olabi, A.G.; Bahri, A.; Abdelghafar, A.A.; Baroutaji, A.; Sayed, E.T.; Alami, A.H.; Rezk, H.; Abdelkareem, M.A. Large-vscale hydrogen production and storage technologies: Current status and future directions. Int. J. Endocrinol. 2021, 46, 23498–23528. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; Bellosta, J.; Blanchard, D.; Bowman, R.C.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P.; et al. Materials for hydrogen-based energy storage e past, recent progress and future outlook. J. Alloys Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Tarhan, C.; Cil, M.A. A study on hydrogen, the clean energy of the future: Hydrogen storage methods. J. Energy Storage 2021, 40, 102676. [Google Scholar] [CrossRef]
- Aakko-Saksa, P.T.; Cook, C.; Kiviaho, J.; Repo, T. Liquid organic hydrogen carriers for transportation and storing of renewable energy-review and discussion. J. Power Sources 2018, 396, 803–823. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, H.; Wang, C.; Ye, E.; Xu, J.W.; Loh, X.J.; Li, Z. Current research progress and perspectives on liquid hydrogen rich molecules in sustainable hydrogen storage. Energy Storage Mater. 2021, 35, 695–722. [Google Scholar] [CrossRef]
- Shen, F.; Smith, R.L., Jr.; Li, J.; Guo, H.; Zhang, X.; Qi, X. Critical assessment of reaction pathways for conversion of agricultural waste biomass into formic acid. Green Chem. 2021, 23, 1536–1561. [Google Scholar] [CrossRef]
- Takagaki, A.; Obata, W.; Ishihara, T. Oxidative conversion of glucose to formic acid as a renewable hydrogen source using an abundant solid base catalyst. Chemistryopen 2021, 10, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.M.; Lien, W.H.; Liao, C.H.; Chen, Y.T.; Huang, S.C.; Chou, F.P.; Chang, C.Y.; Yu, J.S.K.; Kao, Y.T.; Wu, T.K. Efficient and reversible catalysis of formic acid-carbon dioxide cycle using carbamate-substituted ruthenium-dithiolate complexes. ChemCatChem 2021, 13, 4092–4098. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, B.; Wang, N.; He, Q.; Chang, A.; Yang, C.M.; Asakura, H.; Tanaka, T.; Hulsey, M.J.; Wang, C.H.; et al. Zeolite-encaged Pd-Mn nanocatalysts for CO2 hydrogenation and formic acid dehydrogenation. Angew. Chem. Int. Ed. Engl. 2020, 59, 20183–20191. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singh, S.; Kumar, A. Hydrogen energy future with formic acid: A renewable chemical hydrogen storage system. Catal. Sci. Technol. 2016, 6, 12–40. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Q. Metal-nanoparticle-catalyzed hydrogen generation from formic acid. Acc. Chem. Res. 2017, 50, 1449–1458. [Google Scholar] [CrossRef]
- Li, S.; Zhou, Y.; Kang, X.; Liu, D.; Gu, L.; Zhang, Q.; Yan, J.M.; Jiang, Q. A simple and effective principle for a rational design of heterogeneous catalysts for dehydrogenation of formic acid. Adv. Mater. 2019, 31, 1806781. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hu, E.; Yin, G.; Huang, Z. Pd nanoparticles supported on CeO2 nanospheres as efficient catalysts for dehydrogenation from additive-free formic acid at low temperature. Fuel 2021, 302, 121142. [Google Scholar] [CrossRef]
- Li, W.J.; Wey, M.Y. Dual immobilization of Pd Cu nanoparticles on halloysite nanotubes by CTAB and PVP for automobile exhaust elimination. Appl. Clay Sci. 2021, 215, 106312. [Google Scholar] [CrossRef]
- Protasova, L.N.; Rebrov, E.V.; Choy, K.L.; Pung, S.Y.; Engels, V.; Cabaj, M.; Wheatley, A.E.H.; Schouten, J.C. ZnO based nanowires grown by chemical vapour deposition for selective hydrogenation of acetylene alcohols. Catal. Sci. Technol. 2011, 1, 768–777. [Google Scholar] [CrossRef]
- Mori, K.; Dojo, M.; Yamashita, H. Pd and Pd–Ag nanoparticles within a macroreticular basic resin: An efficient catalyst for hydrogen production from formic acid decomposition. ACS Catal. 2013, 3, 1114–1119. [Google Scholar] [CrossRef]
- Tan, H.; Wang, J.; Yu, S.; Zhou, K. Support morphology-dependent catalytic activity of Pd/CeO2 for formaldehyde oxidation. Environ. Sci. Technol. 2015, 49, 8675–8682. [Google Scholar] [CrossRef]
- Jing, H.; Ji, L.; Wang, Z.; Guo, J.; Lu, S.; Sun, J.; Cai, L.; Wang, Y. Synthesis of ZnO nanoparticles loaded on biochar derived from spartina alterniflora with superior photocatalytic degradation performance. Nanomaterials 2021, 11, 2479. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, Q.U.A.; Silveri, F.; Della Pelle, F.; Scroccarello, A.; Zappi, D.; Cozzoni, E.; Compagnone, D. Water-phase exfoliated biocharnanofibers from eucalyptus scraps for electrodemodification and conductive film fabrication. ACS Sustain. Chem. Eng. 2021, 9, 13988–13998. [Google Scholar] [CrossRef]
- Masuda, S.; Mori, K.; Futamura, Y.; Yamashita, H. PdAg nanoparticles supported on functionalized mesoporous carbon: Promotional effect of surface amine groups in reversible hydrogen delivery/storage mediated by formic acid/CO2. AACS Catal. 2018, 8, 2277–2285. [Google Scholar] [CrossRef]
- Lee, D.; Jin, M.; Oh, D.; Lee, S.; Park, J. Straightforward synthesis of hierarchically porous nitrogen-doped carbon via pyrolysis of chitosan/urea/KOH mixtures and its application as a support for formic acid dehydrogenation catalysts. ACS Sustain. Chem. Eng. 2017, 5, 9935–9944. [Google Scholar] [CrossRef]
- Du, W.; Zhang, Z.; Du, L.; Fan, X.; Shen, Z.; Ren, X.; Zhao, Y.; Wei, C.; Wei, S. Designing synthesis of porous biomass carbon from wheat straw and the functionalizing application in flexible, all-solid-state supercapacitors. J. Alloys Compd. 2019, 797, 1031–1040. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, Q.; Gao, S.; Tang, G.; Liu, K.; He, S.; Huang, C. Biomass derived carbon as binder-free electrode materials for supercapacitors. Carbon 2019, 155, 706–726. [Google Scholar] [CrossRef]
- Dubey, P.; Shrivastav, V.; Maheshwari, P.H.; Sundriyal, S. Recent advances in biomass derived activated carbon electrodes for hybrid electrochemical capacitor applications: Challenges and opportunities. Carbon 2020, 170, 1–29. [Google Scholar] [CrossRef]
- Qiu, C.; Chen, Q.; Chang, C.; Jiang, W.; Fan, G. Mechanochemically assisted fabrication of ultrafine Pd nanoparticles on natural waste-derived nitrogen-doped porous carbon for the efficient formic acid decomposition. Int. J. Endocrinol. 2021, 46, 656–665. [Google Scholar] [CrossRef]
- El-Azazy, M.; Nabil, I.; Hassan, S.S.; El-Shafie, A.S. Adsorption characteristics of pristine and magnetic olive stones biochar with respect to clofazimine. Nanomaterials 2021, 11, 963. [Google Scholar] [CrossRef]
- Xu, C.; Stromme, M. Sustainable porous carbon materials derived from wood-based biopolymers for CO2 capture. Nanomaterials 2019, 9, 103. [Google Scholar] [CrossRef] [Green Version]
- Ismail, I.S.; Singh, G.; Smith, P.; Kim, S.; Yang, J.; Joseph, S.; Yusup, S.; Singh, M.; Bansal, V.; Talapaneni, S.N.; et al. Oxygen functionalized porous activated biocarbons with high surface area derived from grape marc for enhanced capture of CO2 at elevated-pressure. Carbon 2020, 160, 113–124. [Google Scholar] [CrossRef]
- Nuilek, K.; Wongwiriyapan, W.; Sattayarut, V.; Simon, A.; Koncz-Horvath, D.; Ferenczi, T.; Kristaly, F.; Baumli, P. Comparison of acid exfoliators in carbon nanosheets synthesis from stinging nettle (urtica dioica) for electrochemical applications. Sci. Rep. 2020, 10, 17270. [Google Scholar] [CrossRef]
- Yi, Y.; Wu, S.; Luo, H.; He, L.; Yang, Y.; Xue, T.; Xu, J.; Wen, Y.; Wang, P. Soft template assisted hydrothermal synthesis of phosphorus doped porous carbon spheres with tunable microstructure as electrochemical nanozyme sensor for distinguishable detection of two flavonoids coupled with derivative voltammetry. J. Electroanal. Chem. 2021, 897, 115563. [Google Scholar] [CrossRef]
- China Statistical Yearbook. Available online: http://www.stats.gov.cn/tjsj/ndsj/2020/indexch.htm (accessed on 2 November 2021).
- Kempasiddaiah, M.; Sree Raj, K.A.; Kandathil, V.; Dateer, R.B.; Sasidhar, B.S.; Yelamaggad, C.V.; Sekhar Rout, C.; Patil, S.A. Waste biomass-derived carbon-supported palladium-based catalyst for cross-coupling reactions and energy storage applications. Appl. Surf. Sci. 2021, 570, 151156. [Google Scholar] [CrossRef]
- Afolalu, S.A.; Salawu, E.Y.; Ogedengbe, T.S.; Joseph, O.O.; Okwilagwe, O.; Emetere, M.E.; Yusuf, O.O.; Noiki, A.A.; Akinlabi, S.A. Bioagro waste valorization and its sustainability in the industry: A review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1107, 012140. [Google Scholar] [CrossRef]
- Lin, F.; Liu, X.; Ma, M.; Qi, F.; Pan, Y.; Wang, L.; Ma, P.; Zhang, Y. Real-time monitoring the carbonization and activation process of activated carbon prepared from Chinese parasol via zinc chloride activation. J. Anal. Appl. Pyrol. 2021, 155, 105089. [Google Scholar] [CrossRef]
- Ozdemir, I.; Şahin, M.; Orhan, R.; Erdem, M. Preparation and characterization of activated carbon from grape stalk by zinc chloride activation. Fuel Process. Technol. 2014, 125, 200–206. [Google Scholar] [CrossRef]
- Sun, L.; Tian, C.; Li, M.; Meng, X.; Wang, L.; Wang, R.; Yin, J.; Fu, H. From coconut shell to porous graphene-like nanosheets for high-power supercapacitors. J. Mater. Chem. A 2013, 1, 6462–6470. [Google Scholar] [CrossRef]
- Jae, J.; Tompsett, G.A.; Foster, A.J.; Hammond, K.D.; Auerbach, S.M.; Lobo, R.F.; Huber, G.W. Investigation into the shape selectivity of zeolite catalysts for biomass conversion. J. Catal. 2011, 279, 257–268. [Google Scholar] [CrossRef]
- Xing, C.; Zhang, Y.; Gao, Y.; Kang, Y.; Zhang, S. N, P co-doped microporous carbon as a metal-free catalyst for the selective oxidation of alcohols by air in water. New J. Chem. 2021, 45, 13877–13884. [Google Scholar] [CrossRef]
- Cao, X.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef] [PubMed]
- Biniak, S.; Szymański, G.; Siedlewski, J.; Świątkowski, A. The characterization of activated carbons with oxygen and nitrogen surface groups. Carbon 1997, 35, 1799–1810. [Google Scholar] [CrossRef]
- Pei, X.; Jiao, H.; Fu, H.; Yin, X.; Luo, D.; Long, S.; Gong, W.; Zhang, L. Facile construction of a highly dispersed Pt nanocatalyst anchored on biomass-derived N/O-doped carbon nanofibrous microspheres and its catalytic hydrogenation. ACS Appl. Mater. Interfaces 2020, 12, 51459–51467. [Google Scholar] [CrossRef]
- Jin, M.; Park, J.; Oh, D.; Lee, S.; Park, J.; Lee, K.; Lee, D. Pd/NH2-KIE-6 catalysts with exceptional catalytic activity for additive-free formic acid dehydrogenation at room temperature: Controlling Pd nanoparticle size by stirring time and types of Pd precursors. Int. J. Endocrinol. 2018, 43, 1451–1458. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Che, H.; Liu, C.; Li, C. Facile construction of O-doped crystalline/non-crystalline g-C3N4 embedded nano-homojunction for efficiently photocatalytic H2 evolution. Carbon 2021, 172, 602–612. [Google Scholar] [CrossRef]
- Song, F.; Wang, X.; Li, T.; Zhang, J.; Bai, Y.; Xing, B.; Giesy, J.P.; Wu, F. Spectroscopic analyses combined with gaussian and coats-redfern models to investigate the characteristics and pyrolysis kinetics of sugarcane residue-derived biochars. J. Clean. Prod. 2019, 237, 117855. [Google Scholar] [CrossRef]
- Nishio, H.; Miura, H.; Kamata, K.; Shishido, T. Deposition of highly dispersed gold nanoparticles onto metal phosphates by deposition–precipitation with aqueous ammonia. Catal. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, S.; Meng, X.; Mao, S.; Lian, X.; Wang, Y. Ultrasmall PdAu alloy nanoparticles anchored on amine-functionalized hierarchically porous carbon as additive-free catalysts for highly efficient dehydrogenation of formic acid. Appl. Catal. B Environ. 2021, 291, 120140. [Google Scholar] [CrossRef]
- Wang, C.; Astruc, D. Recent developments of nanocatalyzed liquid-phase hydrogen generation. Chem. Soc. Rev. 2021, 50, 3437–3484. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, W.; Zhao, H.; Zheng, X.; Wu, L.; Pan, H.; Zhu, J.; Chen, Y.; Lu, J. Size-dependent catalytic activity over carbon-supported palladium nanoparticles in dehydrogenation of formic acid. J. Catal. 2017, 352, 371–381. [Google Scholar] [CrossRef]
- Dolas, H.; Sahin, O.; Saka, C.; Demir, H. A new method on producing high surface area activated carbon: The effect of salt on the surface area and the pore size distribution of activated carbon prepared from pistachio shell. Chem. Eng. J. 2011, 166, 191–197. [Google Scholar] [CrossRef]
- Liu, W.J.; Tian, K.; Jiang, H.; Yu, H.Q. Facile synthesis of highly efficient and recyclable magnetic solid acid from biomass waste. Sci. Rep. 2013, 3, 2419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demirbas, A. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrol. 2004, 72, 243–248. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Noack, A. Black carbon in soils and sediments: Analysis, distribution, implications, and current challenges. Glob. Biogeochem. Cycles 2000, 14, 777–793. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Zacharska, M.; Shlyakhova, E.V.; Chuvilin, A.L.; Guo, Y.; Beloshapkin, S.; Okotrub, A.V.; Bulusheva, L.G. Single isolated Pd2+ Cations Supported on N-doped carbon as active sites for hydrogen production from formic acid decomposition. ACS Catal. 2015, 6, 681–691. [Google Scholar] [CrossRef] [Green Version]
- Jeon, M.; Han, D.J.; Lee, K.S.; Choi, S.H.; Han, J.; Nam, S.W.; Jang, S.C.; Park, H.S.; Yoon, C.W. Electronically modified Pd catalysts supported on N-doped carbon for the dehydrogenation of formic acid. Int. J. Endocrinol. 2016, 41, 15453–15461. [Google Scholar] [CrossRef]







| Catalyst | Average Particle Size (nm) | |
|---|---|---|
| XRD | TEM | |
| Pd/CPS | 7.2 | 6.7 ± 0.6 |
| Pd/CPS-ZnCl2 | 3.8 | 3.6 ± 0.6 |
| Pd/CMS | 6.8 | 6.4 ± 0.4 |
| Pd/CMS-ZnCl2 | 2.8 | 2.9 ± 0.5 |
| Catalyst | SSABET 1 (m2·g−1) | SSAMes 2 (m2·g−1) | SSAMic 3 (m2·g−1) | VT 4 (cm3·g−1) | VMes 5 (cm3·g−1) | VMic 6 (cm3·g−1) |
|---|---|---|---|---|---|---|
| Pd/CPS | 456 | 50 | 406 | 0.23 | 0.03 | 0.20 |
| Pd/CPS-ZnCl2 | 629 | 113 | 517 | 0.34 | 0.09 | 0.25 |
| Pd/CMS | 466 | 50 | 416 | 0.24 | 0.04 | 0.20 |
| Pd/CMS-ZnCl2 | 1081 | 199 | 882 | 0.55 | 0.11 | 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, T.; Cheng, J.; Ma, J.; Yang, C.; Yao, M.; Liu, F.; Deng, M.; Wang, X.; Ren, Y. Facile Synthesis of Microporous Carbons from Biomass Waste as High Performance Supports for Dehydrogenation of Formic Acid. Nanomaterials 2021, 11, 3028. https://doi.org/10.3390/nano11113028
Cao T, Cheng J, Ma J, Yang C, Yao M, Liu F, Deng M, Wang X, Ren Y. Facile Synthesis of Microporous Carbons from Biomass Waste as High Performance Supports for Dehydrogenation of Formic Acid. Nanomaterials. 2021; 11(11):3028. https://doi.org/10.3390/nano11113028
Chicago/Turabian StyleCao, Tingting, Jinke Cheng, Jun Ma, Chunliang Yang, Mengqin Yao, Fei Liu, Min Deng, Xiaodan Wang, and Yuan Ren. 2021. "Facile Synthesis of Microporous Carbons from Biomass Waste as High Performance Supports for Dehydrogenation of Formic Acid" Nanomaterials 11, no. 11: 3028. https://doi.org/10.3390/nano11113028