Organic Dye-Derived N, S Co-Doped Porous Carbon Hosts for Effective Lithium Polysulfide Confinement in Lithium–Sulfur Batteries
Abstract
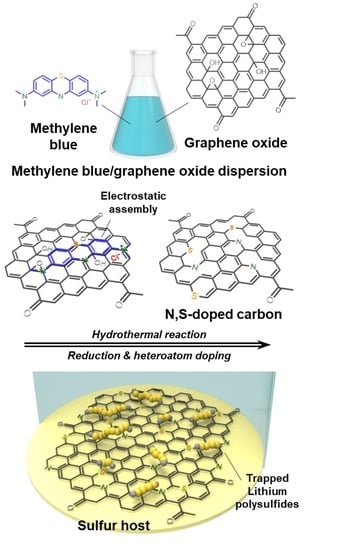
:1. Introduction
2. Materials and Methods
2.1. Preparation of Nitrogen and Sulfur Co-Doped Carbon via Hydrothermal Method
2.2. Material Characterizations
2.3. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, Z.; Li, W.; Wang, Q.; Liu, X. Programmed design of a lithium–sulfur battery cathode by integrating functional units. Adv. Sci. 2019, 6, 1900711. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.X.; Xin, S.; Guo, Y.G.; Wan, L.J. Lithium–sulfur batteries: Electrochemistry, materials, and prospects. Angew. Chem. Inter. Ed. 2013, 52, 13186–13200. [Google Scholar] [CrossRef]
- Eftekhari, A.; Kim, D.-W. Cathode materials for lithium–sulfur batteries: A practical perspective. J. Mater. Chem. A 2017, 5, 17734–17776. [Google Scholar] [CrossRef]
- Song, M.-K.; Cairns, E.J.; Zhang, Y. Lithium/sulfur batteries with high specific energy: Old challenges and new opportunities. Nanoscale 2013, 5, 2186–2204. [Google Scholar] [CrossRef]
- Son, Y.; Lee, J.S.; Son, Y.; Jang, J.H.; Cho, J. Recent advances in lithium sulfide cathode materials and their use in lithium sulfur batteries. Adv. Energy Mater. 2015, 5, 1500110. [Google Scholar] [CrossRef]
- Rehman, S.; Khan, K.; Zhao, Y.; Hou, Y. Nanostructured cathode materials for lithium–sulfur batteries: Progress, challenges and perspectives. J. Mater. Chem. A 2017, 5, 3014–3038. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.; Ahn, H.; Kim, O.; Park, M.J. Synthesis of three-dimensionally interconnected sulfur-rich polymers for cathode materials of high-rate lithium–sulfur batteries. Nat. Commun. 2015, 6, 7278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Sun, J.; Hou, W.; Jiang, S.; Huang, Y.; Geng, J. Three-dimensional porous carbon composites containing high sulfur nanoparticle content for high-performance lithium–sulfur batteries. Nat. Commun. 2016, 7, 10601. [Google Scholar] [CrossRef]
- Guo, J.; Xu, Y.; Wang, C. Sulfur-impregnated disordered carbon nanotubes cathode for lithium–sulfur batteries. Nano Lett. 2011, 11, 4288–4294. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, L.; Zhang, F.; Huang, Y.; Chen, Y. Sulfur-infiltrated graphene-based layered porous carbon cathodes for high-performance lithium–sulfur batteries. ACS Nano 2014, 8, 5208–5215. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Zhu, J.; Yan, C.; Dirican, M.; Zang, J.; Jia, H.; Li, Y.; Kiyak, Y.; Tan, H.; Zhang, X. In situ polymerization of nanostructured conductive polymer on 3D sulfur/carbon nanofiber composite network as cathode for high-performance lithium–sulfur batteries. Adv. Mater. Interfaces 2018, 5, 1701598. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, L.; Yi, Z.; Sun, Y.; Liu, Y.; Jiang, Y.; Shen, Y.; Xin, Y.; Zhang, Z.; Huang, Y. Insight into the electrode mechanism in lithium–sulfur batteries with ordered microporous carbon confined sulfur as the cathode. Adv. Energy Mater. 2014, 4, 1301473. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Zhang, H.; Gao, S.; Han, S.; Xu, Q.; Xu, J.; Lu, W.; Wu, X.; Chen, L. 3D porous spherical sulfur/carbon cathode materials with in situ vapor-phase polymerized polypyrrole coating layer for high-performance lithium–sulfur batteries. ACS Sustain. Chem. Eng. 2019, 7, 17491–17499. [Google Scholar] [CrossRef]
- Peng, H.J.; Hou, T.Z.; Zhang, Q.; Huang, J.Q.; Cheng, X.B.; Guo, M.Q.; Yuan, Z.; He, L.Y.; Wei, F. Strongly coupled interfaces between a heterogeneous carbon host and a sulfur-containing guest for highly stable lithium–sulfur batteries: Mechanistic insight into capacity degradation. Adv. Mater. Interfaces 2014, 1, 1400227. [Google Scholar] [CrossRef]
- Li, G.C.; Li, G.R.; Ye, S.H.; Gao, X.P. A polyaniline-coated sulfur/carbon composite with an enhanced high-rate capability as a cathode material for lithium/sulfur batteries. Adv. Energy Mater. 2012, 2, 1238–1245. [Google Scholar] [CrossRef]
- Sun, Q.; Xi, B.; Li, J.Y.; Mao, H.; Ma, X.; Liang, J.; Feng, J.; Xiong, S. Nitrogen-Doped Graphene-Supported Mixed Transition-Metal Oxide Porous Particles to Confine Polysulfides for Lithium–Sulfur Batteries. Adv. Energy Mater. 2018, 8, 1800595. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, J.; Yin, L.; Hu, G.; Fang, R.; Cheng, H.-M.; Li, F. Conductive porous vanadium nitride/graphene composite as chemical anchor of polysulfides for lithium–sulfur batteries. Nat. commun. 2017, 8, 14627. [Google Scholar] [CrossRef]
- Razaq, R.; Sun, D.; Xin, Y.; Li, Q.; Huang, T.; Zhang, Z.; Huang, Y. Nanoparticle Assembled Mesoporous MoO2 Microrods Derived from Metal Organic Framework and Wrapped with Graphene as the Sulfur Host for Long-Life Lithium–Sulfur Batteries. Adv. Mater. Interfaces 2019, 6, 1801636. [Google Scholar] [CrossRef]
- Hou, T.Z.; Chen, X.; Peng, H.J.; Huang, J.Q.; Li, B.Q.; Zhang, Q.; Li, B. Design principles for heteroatom-doped nanocarbon to achieve strong anchoring of polysulfides for lithium–sulfur batteries. Small 2016, 12, 3283–3291. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Zhao, Y.; Manthiram, A. Dual-confined flexible sulfur cathodes encapsulated in nitrogen-doped double-shelled hollow carbon spheres and wrapped with graphene for Li–S batteries. Adv. Energy Mater. 2015, 5, 1402263. [Google Scholar] [CrossRef]
- Song, J.; Gordin, M.L.; Xu, T.; Chen, S.; Yu, Z.; Sohn, H.; Lu, J.; Ren, Y.; Duan, Y.; Wang, D. Strong lithium polysulfide chemisorption on electroactive sites of nitrogen-doped carbon composites for high-performance lithium–sulfur battery cathodes. Angew. Chem. Inter. Ed. 2015, 127, 4399–4403. [Google Scholar] [CrossRef]
- Pang, Q.; Tang, J.; Huang, H.; Liang, X.; Hart, C.; Tam, K.C.; Nazar, L.F. A nitrogen and sulfur dual-doped carbon derived from polyrhodanine@ cellulose for advanced lithium–sulfur batteries. Adv. Mater. 2015, 27, 6021–6028. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, S.; Jiang, S.; Huang, C.; Wang, X.; Yang, Z.; Xiang, K.; Zhang, Y. Suppressing the polysulfide shuttle effect by heteroatom-doping for high-performance lithium–sulfur batteries. ACS Sustain. Chem. Eng. 2018, 6, 7545–7557. [Google Scholar] [CrossRef]
- Ngidi, N.P.; Ollengo, M.A.; Nyamori, V.O. Effect of doping temperatures and nitrogen precursors on the physicochemical, optical, and electrical conductivity properties of nitrogen-doped reduced graphene oxide. Materials 2019, 12, 3376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.; Dou, S.; Shen, A.; Tao, L.; Dai, L.; Wang, S. Sulfur-Doped Graphene Derived from Cycled Lithium–Sulfur Batteries as a Metal-Free Electrocatalyst for the Oxygen Reduction Reaction. Angew. Chem. Inter. Ed. 2015, 54, 1888–1892. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, Y.; Du, H.; He, S.; Liu, L.; Fu, Z.; Xie, L.; Ai, W.; Huang, W. Molecularly designed N, S co-doped carbon nanowalls decorated on graphene as a highly efficient sulfur reservoir for Li–S batteries: A supramolecular strategy. J. Mater. Chem. A 2020, 8, 5449–5457. [Google Scholar] [CrossRef]
- Zhou, X.; Tian, J.; Wu, Q.; Hu, J.; Li, C. N/O dual-doped hollow carbon microspheres constructed by holey nanosheet shells as large-grain cathode host for high loading Li-S batteries. Energy Storage Mater. 2020, 24, 644–654. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, W.; Zhao, W.; Li, G.; Hou, Y.; Liu, M.; Zhou, L.; Ye, F.; Li, H.; Wei, Z. High-rate, ultralong cycle-life lithium/sulfur batteries enabled by nitrogen-doped graphene. Nano Lett. 2014, 14, 4821–4827. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Tu, W.; Pan, H.; Zhang, H.; Liu, B.; Cheng, Y.; Deng, Z.; Zhang, H. Self-templating synthesis of hollow Co3O4 nanoparticles embedded in N, S-dual-doped reduced graphene oxide for lithium ion batteries. ACS Nano 2020, 14, 5780–5787. [Google Scholar] [CrossRef] [PubMed]
- Ai, W.; Zhou, W.; Du, Z.; Chen, Y.; Sun, Z.; Wu, C.; Zou, C.; Li, C.; Huang, W.; Yu, T. Nitrogen and phosphorus codoped hierarchically porous carbon as an efficient sulfur host for Li-S batteries. Energy Storage Mater. 2017, 6, 112–118. [Google Scholar] [CrossRef]
- Yao, W.; Zheng, W.; Xu, J.; Tian, C.; Han, K.; Sun, W.; Xiao, S. ZnS-SnS@ NC Heterostructure as robust lithiophilicity and sulfiphilicity mediator toward high-rate and long-life lithium–sulfur batteries. ACS Nano 2021, 15, 7114–7130. [Google Scholar] [CrossRef] [PubMed]
- Kasnatscheew, J.; Evertz, M.; Streipert, B.; Wagner, R.; Klöpsch, R.; Vortmann, B.; Hahn, H.; Nowak, S.; Amereller, M.; Gentschev, A.-C. The truth about the 1st cycle Coulombic efficiency of LiNi1/3Co1/3Mn1/3O2 (NCM) cathodes. Phys. Chem. Chem. Phys. 2016, 18, 3956–3965. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kang, J.; Kim, S.-W.; Halim, W.; Frey, M.W.; Joo, Y.L. Effective suppression of the polysulfide shuttle effect in lithium–sulfur batteries by implementing rGO–PEDOT: PSS-coated separators via air-controlled electrospray. ACS Omega 2018, 3, 16465–16471. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Lv, W.; Zhou, G.; He, Y.; Li, B.; Yang, Q.-H.; Kang, F. N and S co-doped porous carbon spheres prepared using l-cysteine as a dual functional agent for high-performance lithium–sulfur batteries. Chem. Commun. 2015, 51, 17720–17723. [Google Scholar] [CrossRef]
- Lu, L.; Pei, F.; Abeln, T.; Pei, Y. Tailoring three-dimensional interconnected nanoporous graphene micro/nano-foams for lithium–sulfur batteries. Carbon 2020, 157, 437–447. [Google Scholar] [CrossRef]
- Chen, S.; Ming, Y.; Tan, B.; Chen, S. Carbon-free sulfur-based composite cathode for advanced Lithium–Sulfur batteries: A case study of hierarchical structured CoMn2O4 hollow microspheres as sulfur immobilizer. Electrochim. Acta 2020, 329, 135128. [Google Scholar] [CrossRef]
- Li, M.; Zhou, X.; Ma, X.; Chen, L.; Zhang, D.; Xu, S.; Duan, D.; Chen, C.; Yuan, Q.; Liu, S. Development of sulfonated-carbon nanotubes/graphene three-dimensional conductive spongy framework with ion-selective effect as cathode in high-performance lithium–sulfur batteries. Chem. Eng. J. 2021, 409, 128164. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Z.; Cao, T.; Qin, K.; Xu, Q.; Liu, H.; Xia, Y. Multishelled Ni2P microspheres as multifunctional sulfur host 3D-printed cathode materials ensuring high areal capacity of lithium–sulfur datteries. ACS Sustain. Chem. Eng. 2021, 9, 6097–6106. [Google Scholar] [CrossRef]
- Wang, X.; Gao, T.; Han, F.; Ma, Z.; Zhang, Z.; Li, J.; Wang, C. Stabilizing high sulfur loading Li–S batteries by chemisorption of polysulfide on three-dimensional current collector. Nano Energy 2016, 30, 700–708. [Google Scholar] [CrossRef] [Green Version]
- Su, D.; Cortie, M.; Wang, G. Fabrication of N-doped graphene–carbon nanotube hybrids from Prussian blue for lithium–sulfur batteries. Adv. Energy Mater. 2017, 7, 1602014. [Google Scholar] [CrossRef]
- Bao, W.; Liu, L.; Wang, C.; Choi, S.; Wang, D.; Wang, G. Facile synthesis of crumpled nitrogen-doped mxene nanosheets as a new sulfur host for lithium–sulfur batteries. Adv. Energy Mater. 2018, 8, 1702485. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.; Kim, J.; Lee, T.; Kang, H.; Yu, S.; Park, J.W.; Lee, S.G.; Li, O.L.; Lee, J.H. Plasma-engineered organic dyes as efficient polysulfide-mediating layers for high performance lithium–sulfur batteries. Chem. Eng. J. 2021, 430, 132679. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.; Lee, A.S.; Lee, T.; Seo, H.J.; Chae, S.; Kim, K.; Park, J.-W.; Lee, S.G.; Lee, J.H. Organic Dye-Derived N, S Co-Doped Porous Carbon Hosts for Effective Lithium Polysulfide Confinement in Lithium–Sulfur Batteries. Nanomaterials 2021, 11, 2954. https://doi.org/10.3390/nano11112954
Kim E, Lee AS, Lee T, Seo HJ, Chae S, Kim K, Park J-W, Lee SG, Lee JH. Organic Dye-Derived N, S Co-Doped Porous Carbon Hosts for Effective Lithium Polysulfide Confinement in Lithium–Sulfur Batteries. Nanomaterials. 2021; 11(11):2954. https://doi.org/10.3390/nano11112954
Chicago/Turabian StyleKim, Eunji, Albert S. Lee, Taewoong Lee, Hyeok Jun Seo, Seongwook Chae, Kihyun Kim, Jun-Woo Park, Seung Geol Lee, and Jin Hong Lee. 2021. "Organic Dye-Derived N, S Co-Doped Porous Carbon Hosts for Effective Lithium Polysulfide Confinement in Lithium–Sulfur Batteries" Nanomaterials 11, no. 11: 2954. https://doi.org/10.3390/nano11112954