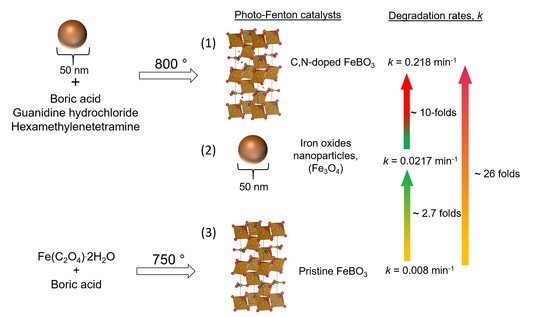
Facile Synthesis of Carbon- and Nitrogen-Doped Iron Borate as a Highly Efficient Single-Component Heterogeneous Photo-Fenton Catalyst under Simulated Solar Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the C,N-Doped Iron Borate
2.2. Characterization
2.3. Photocatalytic Degradation Experiments
2.4. Active Species Analysis
3. Results
Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayak, A.; Agarwal, S. Chemical treatment technologies for waste-water recycling—An overview. RSC Adv. 2012, 2, 6380. [Google Scholar] [CrossRef]
- Mandal, T.; Maity, S.; Dasgupta, D.; Datta, S. advanced oxidation process and biotreatment: Their roles in combined industrial wastewater treatment. Desalination 2010, 250, 87–94. [Google Scholar] [CrossRef]
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton catalysts: A review of recent advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhu, J.; Zhu, G.; He, H. Strategies for enhancing the heterogeneous fenton catalytic reactivity: A review. Appl. Catal. B Environ. 2019, 255, 117739. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef]
- Bauer, R. The photo-fenton reaction and the TiO2/UV process for waste water treatment—Novel developments. Catal. Today 1999, 53, 131–144. [Google Scholar] [CrossRef]
- Giannakis, S.; López, M.I.P.; Spuhler, D.; Pérez, J.A.S.; Ibáñez, P.F.; Pulgarin, C. Solar disinfection is an augmentable, in situ-generated photo-fenton reaction—Part 2: A review of the applications for drinking water and wastewater disinfection. Appl. Catal. B Environ. 2016, 198, 431–446. [Google Scholar] [CrossRef]
- Lamb, J.J.; Islam, M.H.; Hjelme, D.R.; Pollet, B.G.; Lien, K.M. Effect of power ultrasound and fenton reagents on the biomethane potential from steam-exploded birchwood. Ultrason. Sonochem. 2019, 58, 104675. [Google Scholar] [CrossRef]
- Ning, X.; Chen, H.; Wu, J.; Wang, Y.; Liu, J.; Lin, M. Effects of ultrasound assisted fenton treatment on textile dyeing sludge structure and dewaterability. Chem. Eng. J. 2014, 242, 102–108. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, L.; Liu, L.; Li, J.; Wang, S.; Znad, H.; Liu, S. Magnetic ZnO@Fe3O4 composite for self-generated H2O2 toward photo-fenton-like oxidation of nitrophenol. Compos. Part B Eng. 2020, 200, 108345. [Google Scholar] [CrossRef]
- Sun, Q.; Hong, Y.; Liu, Q.; Dong, L. Synergistic operation of photocatalytic degradation and fenton process by magnetic Fe3O4 loaded TiO2. Appl. Surf. Sci. 2018, 430, 399–406. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, Q.; Wu, A.; Jiang, M.; Liang, Z.; Jiang, B.; Fu, H. Cost-effective large-scale synthesis of ZnO photocatalyst with excellent performance for dye photodegradation. Chem. Commun. 2012, 48, 2858. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ma, C.; Liu, Y.; Xing, L.; Yan, Y. Self-induced fenton reaction constructed by Fe(III) grafted BiVO4 nanosheets with improved photocatalytic performance and mechanism insight. Appl. Surf. Sci. 2019, 467–468, 673–683. [Google Scholar] [CrossRef]
- Huang, T.; Zhu, J.; Ge, S.; Guo, T.; Jiang, C.; Xie, L. Synthesis of novel CdSe QDs/BiFeO3 composite catalysts and its application for the photo-fenton catalytic degradation of phenol. J. Environ. Chem. Eng. 2020, 8, 104384. [Google Scholar] [CrossRef]
- Ma, J.; Yang, Q.; Wen, Y.; Liu, W. Fe-g-C3N4/graphitized mesoporous carbon composite as an effective fenton-like catalyst in a wide PH range. Appl. Catal. B Environ. 2017, 201, 232–240. [Google Scholar] [CrossRef]
- Diehl, R. Crystal structure refinement of ferric borate, FeBO3. Solid State Commun. 1975, 17, 743–745. [Google Scholar] [CrossRef]
- Joubert, J.C.; Shirk, T.; White, W.B.; Roy, R. Stability, infrared spectrum and magnetic properties of FeBO3. Mater. Res. Bull. 1968, 3, 671–676. [Google Scholar] [CrossRef]
- Yagupov, S.; Strugatsky, M.; Seleznyova, K.; Mogilenec, Y.; Snegirev, N.; Marchenkov, N.V.; Kulikov, A.G.; Eliovich, Y.A.; Frolov, K.V.; Ogarkova, Y.L.; et al. Development of a synthesis technique and characterization of high-quality iron borate FeBO3 single crystals for applications in synchrotron technologies of a new generation. Cryst. Growth Des. 2018, 18, 7435–7440. [Google Scholar] [CrossRef]
- Dastafkan, K.; Li, Y.; Zeng, Y.; Han, L.; Zhao, C. Enhanced surface wettability and innate activity of iron borate catalyst for efficient oxygen evolution and gas bubble detachment. J. Mater. Chem. A 2019, 7, 15252–15261. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, T.; Li, T.; Wang, Y.; Liu, H.; Feng, J.; Ding, S.; Li, Z.; Wu, M. Amorphous iron(III)-borate nanolattices as multifunctional electrodes for self-driven overall water splitting and rechargeable zinc-air battery. Small 2018, 14, 1802829. [Google Scholar] [CrossRef]
- Huang, C.; Chen, C.; Zhang, M.; Lin, L.; Ye, X.; Lin, S.; Antonietti, M.; Wang, X. Carbon-doped BN nanosheets for metal-free photoredox catalysis. Nat. Commun. 2015, 6, 7698. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Wu, C.; Kim, D.-H.; Lee, B.W.; Rhee, S.J.; Park, Y.C.; Kim, C.S.; Wang, Q.J.; Liu, C. Nitrogen doped BiFeO3 with enhanced magnetic properties and photo-fenton catalytic activity for degradation of bisphenol a under visible light. Chem. Eng. J. 2018, 337, 709–721. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, H.; Qin, J.; Wu, G.; Lian, C.; Zhang, J.; Wang, S. Iron encapsulated in boron and nitrogen codoped carbon nanotubes as synergistic catalysts for fenton-like reaction. Water Res. 2016, 101, 281–291. [Google Scholar] [CrossRef]
- Yu, J.; Chen, Z.; Zeng, L.; Ma, Y.; Feng, Z.; Wu, Y.; Lin, H.; Zhao, L.; He, Y. Synthesis of carbon-doped KNbO3 photocatalyst with excellent performance for photocatalytic hydrogen production. Sol. Energy Mater. Sol. Cells 2018, 179, 45–56. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, S.; Zhou, Y.; Yang, Y.; Zhang, J.; Luo, L.; Duan, X.; Wang, S.; Wang, L.; Tsang, D.C.W. Insights into the oxidation of organic contaminants by iron nanoparticles encapsulated within boron and nitrogen co-doped carbon nanoshell: Catalyzed fenton-like reaction at natural PH. Environ. Int. 2019, 128, 77–88. [Google Scholar] [CrossRef]
- Ogi, T.; Iwasaki, H.; Nandiyanto, A.B.D.; Iskandar, F.; Wang, W.-N.; Okuyama, K. Direct white light emission from a rare-earth-free aluminium–boron–carbon–oxynitride phosphor. J. Mater. Chem. C 2014, 2, 4297–4303. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, M.; Zhang, Y.; Gu, N. Synthesis of nanometer-size maghemite particles from magnetite. Colloids Surf. A Physicochem. Eng. Asp. 2004, 245, 15–19. [Google Scholar] [CrossRef]
- Das, S.K.; Nandi, M.; Giri, S.; Bhaumik, A. A new mesoporous FeBO3 material having dominant surface magnetism. Microporous Mesoporous Mater. 2009, 117, 362–367. [Google Scholar] [CrossRef]
- Kumari, K.; Ram, S.; Kotnala, R.K. Self-controlled growth of Fe3BO6 crystallites in shape of nanorods from iron-borate glass of small templates. Mater. Chem. Phys. 2011, 129, 1020–1026. [Google Scholar] [CrossRef]
- Singh, B.; Kaur, G.; Singh, P.; Singh, K.; Kumar, B.; Vij, A.; Kumar, M.; Bala, R.; Meena, R.; Singh, A.; et al. Nanostructured boron nitride with high water dispersibility for boron neutron capture therapy. Sci. Rep. 2016, 6, 35535. [Google Scholar] [CrossRef] [Green Version]
- Sudeep, P.M.; Vinod, S.; Ozden, S.; Sruthi, R.; Kukovecz, A.; Konya, Z.; Vajtai, R.; Anantharaman, M.R.; Ajayan, P.M.; Narayanan, T.N. Functionalized boron nitride porous solids. RSC Adv. 2015, 5, 93964–93968. [Google Scholar] [CrossRef]
- Zhang, C.C.; Gao, X.; Yilmaz, B. Development of FTIR spectroscopy methodology for characterization of boron species in FCC catalysts. Catalysts 2020, 10, 1327. [Google Scholar] [CrossRef]
- Yumura, T.; Amenomori, T.; Kagawa, Y.; Yoshizawa, K. Mechanism for the formaldehyde to formic acid and the formic acid to carbon dioxide conversions mediated by an iron-oxo species. J. Phys. Chem. A 2002, 106, 621–630. [Google Scholar] [CrossRef]
- Busca, G.; Lorenzelli, V. Infrared study of CO2 adsorption on haematite. Mater. Chem. 1980, 5, 213–223. [Google Scholar] [CrossRef]
- Liu, R.; Ma, Z.; Sears, J.D.; Juneau, M.; Neidig, M.L.; Porosoff, M.D. Identifying correlations in fischer-tropsch synthesis and CO2 hydrogenation over Fe-based ZSM-5 catalysts. J. CO2 Util. 2020, 41, 101290. [Google Scholar] [CrossRef]
- Starobrat, A.; Jaroń, T.; Grochala, W. New hydrogen-rich ammonium metal borohydrides, NH4[M(BH4)4], M = Y, Sc, Al, as potential H2 sources. Dalton Trans. 2018, 47, 4442–4448. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Hong, S.-A.; Son, H.-J.; Han, W.-S.; Michalak, A.; Hwang, S.-J.; Kang, S.O. Dehydrogenation of ammonia-borane by cationic Pd(II) and Ni(II) complexes in a nitromethane medium: Hydrogen release and spent fuel characterization. Dalton Trans. 2015, 44, 7373–7381. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, K.; Cruz-Silva, R.; Yang, K.-S.; Kim, Y.A.; Hayashi, T.; Endo, M.; Terrones, M.; Dresselhaus, M.S. Importance of open, heteroatom-decorated edges in chemically doped-graphene for supercapacitor applications. J. Mater. Chem. A 2014, 2, 9532–9540. [Google Scholar] [CrossRef]
- Kurmaev, E.; Fedorenko, V.; Galakhov, V.; Bartkowski, S.; Uhlenbrock, S.; Neumann, M.; Slater, P.; Greaves, C.; Miyazaki, Y. Analysis of oxyanion (BO33−, CO32−, SO42−, PO43−, SeO44−) substitution in Y123 compounds studied by X-ray photoelectron spectroscopy. J. Supercond. 1996, 9, 97–100. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, P.; An, W.; Liu, L.; Liang, Y.; Cui, W. In-situ Fe-doped g-C3N4 heterogeneous catalyst via photocatalysis-fenton reaction with enriched photocatalytic performance for removal of complex wastewater. Appl. Catal. B Environ. 2019, 245, 130–142. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Lyubutin, I.; Sarkisyan, V.; Gavriliuk, A.; Troyan, I.; Rüffer, R. Hyperfine interactions and electronic structure of FeBO3 at high pressure. Bull. Russ. Acad. Sci. Phys. 2003, 67, 1018. [Google Scholar]
- Wu, B.; Qi, S.; Wu, X.; Wang, H.; Zhuang, Q.; Yi, H.; Xu, P.; Xiong, Z.; Shi, G.; Chen, S.; et al. FeBO3 as a low cost and high-performance anode material for sodium-ion batteries. In press. Chin. Chem. Lett. 2021. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Y.; Xia, X.; Wang, L.; Shao, L.; Cai, T.; Dong, W. Mechanisms for photo assisted fenton of synthesized pyrrhotite at neutral PH. Appl. Surf. Sci. 2019, 463, 863–871. [Google Scholar] [CrossRef]
- Bahrudin, N.N.; Nawi, M.A.; Nawawi, W.I. Photocatalytic enhancement of immobilized TiO2-polyaniline bilayer (TiO2-PBL) system for decolorization of methyl orange dye. Mater. Res. Bull. 2018, 106, 388–395. [Google Scholar] [CrossRef]
- Huang, H.; He, Y.; Lin, Z.; Kang, L.; Zhang, Y. Two novel bi-based borate photocatalysts: Crystal structure, electronic structure, photoelectrochemical properties, and photocatalytic activity under simulated solar light irradiation. J. Phys. Chem. C 2013, 117, 22986–22994. [Google Scholar] [CrossRef]
- Li, Y.; Diao, Y.; Wang, X.; Tian, X.; Hu, Y.; Zhang, B.; Yang, D. Zn4 B6 O13: Efficient borate photocatalyst with fast carrier separation for photodegradation of tetracycline. Inorg. Chem. 2020, 59, 13136–13143. [Google Scholar] [CrossRef]
- Hu, X.; Li, R.; Zhao, S.; Xing, Y. Microwave-assisted preparation of flower-like cobalt phosphate and its application as a new heterogeneous fenton–like catalyst. Appl. Surf. Sci. 2017, 396, 1393–1402. [Google Scholar] [CrossRef]
- Rajoriya, S.; Bargole, S.; George, S.; Saharan, V.K.; Gogate, P.R.; Pandit, A.B. Synthesis and characterization of samarium and nitrogen doped TiO2 photocatalysts for photo-degradation of 4-acetamidophenol in combination with hydrodynamic and acoustic cavitation. Sep. Purif. Technol. 2019, 209, 254–269. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, S.-Y.; Lin, E.-X.; Keng, P.-Y. Facile Synthesis of Carbon- and Nitrogen-Doped Iron Borate as a Highly Efficient Single-Component Heterogeneous Photo-Fenton Catalyst under Simulated Solar Irradiation. Nanomaterials 2021, 11, 2853. https://doi.org/10.3390/nano11112853
Hsiao S-Y, Lin E-X, Keng P-Y. Facile Synthesis of Carbon- and Nitrogen-Doped Iron Borate as a Highly Efficient Single-Component Heterogeneous Photo-Fenton Catalyst under Simulated Solar Irradiation. Nanomaterials. 2021; 11(11):2853. https://doi.org/10.3390/nano11112853
Chicago/Turabian StyleHsiao, Shan-Yuan, En-Xuan Lin, and Pei-Yuin Keng. 2021. "Facile Synthesis of Carbon- and Nitrogen-Doped Iron Borate as a Highly Efficient Single-Component Heterogeneous Photo-Fenton Catalyst under Simulated Solar Irradiation" Nanomaterials 11, no. 11: 2853. https://doi.org/10.3390/nano11112853