Facile Preparation and Characteristic Analysis of Sulfated Cellulose Nanofibril via the Pretreatment of Sulfamic Acid-Glycerol Based Deep Eutectic Solvents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. DES Sulfated Pretreatment
2.3. Nano-Fibrillation of Sulfated Cellulose
2.4. Preparation of Neat PVA and PVA/CNF Films
2.5. Characterizations
2.5.1. Characterizations of Sulfated Pulp
2.5.2. Characterizations of Sulfated CNF
2.5.3. Characterizations of PVA/CNF Films
3. Results and Discussion
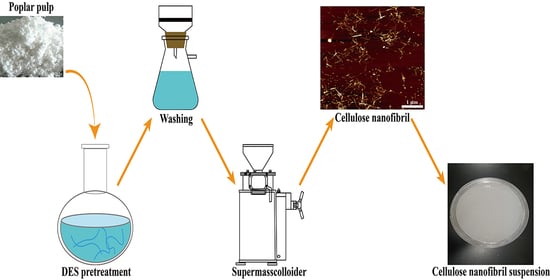
3.1. Preparation of Sulfated CNF via DES
3.2. Characteristics of Sulfated Fibers
3.2.1. The Yields and Fiber Width of Sulfated Fibers
3.2.2. The Degree of Polymerization of Sulfated Fibers
3.2.3. The FTIR and Elemental Analysis Sulfated Fibers
3.3. Characterization of Cellulose Nanofibril
3.3.1. Water Retention Value and Energy Consumption of Cellulose Nanofibril
3.3.2. The Preparation Mechanism of Sulfated Cellulose Nanofibril
3.3.3. The Morphology and Size Dimensions of Cellulose Nanofibril
3.3.4. The Zeta Potential and Crystallinity of Cellulose Nanofibril
3.4. Characterization of Neat PVA and PVA/CNF Films
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ling, S.; Chen, W.; Fan, Y.; Zheng, K.; Jin, K.; Yu, H.; Buehler, M.J.; Kaplan, D.L. Biopolymer nanofibrils: Structure, modeling, preparation, and applications. Prog. Polym. Sci. 2018, 85, 1–56. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.H.; Ma, G.R.; He, M.; Ji, X.X.; Youn, H.J.; Lee, H.L.; Chen, J.C. Application of cellulose nanofibril as a wetend additive in papermaking: A brief review. Paper Biomater. 2020, 5, 76–84. [Google Scholar]
- Tayeb, P.; Tayeb, A.H. Nanocellulose applications in sustainable electrochemical and piezoelectric systems: A review. Carbohydr. Polym. 2019, 224, 115149. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, M.M.S.; Elyas, A.; Mehdi, T.; Bousfield, D.W.; Mohammadreza, D.F. Application of cellulose nanofibril (CNF) as coating on paperboard at moderate solids content and high coating speed using blade coater. Prog. Org. Coat. 2018, 122, 207–218. [Google Scholar]
- Curvello, R.; Raghuwanshi, V.S.; Garnier, G. Engineering nanocellulose hydrogels for biomedical applications. Adv. Colloid Interface Sci. 2019, 267, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Li, T.; Li, Y.J.; Zhang, Y.; Gong, A.; Dai, J.Q.; Hitz, E.; Luo, W.; Hu, L.B. Wood-based nanotechnologies toward sus-tainability. Adv. Mater. 2018, 30, 1703453–1703492. [Google Scholar] [CrossRef]
- He, M.; Yang, G.; Chen, J.; Ji, X.; Wang, Q. Production and Characterization of Cellulose Nanofibrils from Different Chemical and Mechanical Pulps. J. Wood Chem. Technol. 2018, 38, 149–158. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Lee, H.; Sundaram, J.; Zhu, L.; Zhao, Y.; Mani, S. Improved thermal stability of cellulose nanofibrils using low-concentration alkaline pretreatment. Carbohydr. Polym. 2018, 181, 506–513. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Lin, X.J.; Zhang, N.; Lu, Y.X.; Wu, Z.M.; Liu, G.L.; Nie, S.X. Chemically functionalized cellulose nano-fibrils-based gear-like triboelectric nanogenerator for energy harvesting and sensing. Nano Energy 2019, 66, 104126–104136. [Google Scholar] [CrossRef]
- Im, W.; Oh, K.; Abhari, A.R.; Youn, H.J.; Lee, H.L. Recycling of isopropanol for cost-effective, environmentally friendly pro-duction of carboxymethylated cellulose nanofibrils. Carbohydr. Polym. 2019, 208, 365–371. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Q.; Gou, S.; Zhang, L.; Wang, Z. Esterification of cellulose using carboxylic acid-based deep eutectic solvents to produce high-yield cellulose nanofibers. Carbohydr. Polym. 2021, 251, 117018. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Li, H.; Fu, S.; Lucia, L.A. The non-trivial role of native xylans on the preparation of TEMPO-oxidized cellulose nanofibrils. React. Funct. Polym. 2014, 85, 142–150. [Google Scholar] [CrossRef]
- Weber, D.; Knaak, S.; Hettrich, K.; Andrulis, M.; Momburg, F.; Quade, M.; Gelinsky, M.; Schwartz-Albiez, R. Influence of Regioselectively Sulfated Cellulose on in Vitro Vascularization of Biomimetic Bone Matrices. Biomacromolecules 2018, 19, 4228–4238. [Google Scholar] [CrossRef]
- Chang, H.; Luo, J.; Davijani, A.A.B.; Chien, A.-T.; Wang, P.-H.; Liu, H.C.; Kumar, S. Individually Dispersed Wood-Based Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2016, 8, 5768–5771. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Du, H.; Liu, K.; Liu, H.; Xu, T.; Zhang, S.; Chen, X.; Zhang, R.; Li, H.; Xie, H.; et al. Sustainable preparation of bifunctional cellulose nanocrystals via mixed H2SO4/formic acid hydrolysis. Carbohydr. Polym. 2021, 266, 118107. [Google Scholar] [CrossRef] [PubMed]
- Rånby, B.G.; Banderet, A.; Sillén, L.G. Aqueous Colloidal Solutions of Cellulose Micelles. Acta Chem. Scand. 1949, 3, 649–650. [Google Scholar] [CrossRef] [Green Version]
- Svensson, A.; Nicklasson, E.; Harrah, T.; Panilaitis, B.; Kaplan, D.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential scaffoldfor tissue engineering of cartilage. Biomaterials 2005, 26, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Peschel, D.; Bäucker, E.; Groth, T.; Fischer, S. Synthesis and characterisation of cellulose sulfates regarding the degrees of substitution, degrees of polymerisation and morphology. Carbohydr. Polym. 2011, 83, 1659–1664. [Google Scholar] [CrossRef]
- Luo, J.; Semenikhin, N.; Chang, H.; Moon, R.J.; Kumar, S. Post-sulfonation of cellulose nanofibrils with a one-step reaction to improve dispersibility. Carbohydr. Polym. 2018, 181, 247–255. [Google Scholar] [CrossRef]
- Scelsi, E.; Angelini, A.; Pastore, C. Deep eutectic solvents for the valorisation of lignocellulosic biomasses towards Fine Chemicals. Biomass 2021, 1, 29–59. [Google Scholar] [CrossRef]
- Nguyen, H.V.D.; Vries, R.D.; Stoyanov, S.D. Natural deep eutectics as “green” cellulose cosolvent. ACS Sustain. Chem. Eng. 2020, 8, 14166–14178. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2020, 121, 1232–1285. [Google Scholar] [CrossRef]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jerome, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Asoh, T.-A.; Uyama, H. Cationic functionalization of cellulose monoliths using a urea-choline based deep eutectic solvent and their applications. Polym. Degrad. Stab. 2019, 160, 126–135. [Google Scholar] [CrossRef]
- Liu, S.; Edgar, K.J. Staudinger Reactions for Selective Functionalization of Polysaccharides: A Review. Biomacromolecules 2015, 16, 2556–2571. [Google Scholar] [CrossRef] [PubMed]
- Willberg-Keyriläinen, P.; Hiltunen, J.; Ropponen, J. Production of cellulose carbamate using urea-based deep eutectic solvents. Cellulose 2017, 25, 195–204. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Ukkola, J.; Liimatainen, H. Direct sulfation of cellulose fibers using a reactive deep eutectic solvent to produce highly charged cellulose nanofibers. Cellulose 2019, 26, 2303–2316. [Google Scholar] [CrossRef] [Green Version]
- Yuliana, M.; Trisna, L.; Sari, F.; Lunardi, V.B. Glycerol purification using reactivated spent bleaching earth from palm oil re-fineries: Zero-waste approach. J. Environ. Chem. Eng. 2021, 9, 105239. [Google Scholar] [CrossRef]
- Yu, W.; Wang, C.; Yi, Y.; Wang, H.; Zeng, L.; Li, M.; Yang, Y.; Tan, Z. Comparison of Deep Eutectic Solvents on Pretreatment of Raw Ramie Fibers for Cellulose Nanofibril Production. ACS Omega 2020, 5, 5580–5588. [Google Scholar] [CrossRef]
- Hou, Q.; Wang, X.; Ragauskas, A.J. Preparation and characterization of nanocellulose–polyvinyl alcohol multilayer film by layer-by-layer method. Cellulose 2019, 26, 4787–4798. [Google Scholar] [CrossRef]
- Peng, H.; Wang, S.; Xu, H.Y.; Hao, X. Preparations and properties and the composite mechanism of cellulose/polyvinyl alcohol bio-composite hydrogel membranes. New J. Chem. 2017, 41, 6564–6573. [Google Scholar] [CrossRef]
- Kučan, K.Z.; Perković, M.; Cmrk, K.; Načinović, D.; Rogošić, M. Betaine + (glycerol or ethylene glycol or propylene glycol) deep eutectic solvents for extractive purification of gasoline. Chem. Select 2018, 3, 12582–12590. [Google Scholar] [CrossRef]
- Skulcova, A.; Russ, A.; Jablonsky, M.; Sima, J. The pH behavior of seventeen deep eutectic solvents. BioResources 2018, 13, 5042–5051. [Google Scholar]
- Liimatainen, H.; Sirviö, J.; Haapala, A.; Hormi, O.; Niinimäki, J. Characterization of highly accessible cellulose microfibers generated by wet stirred media milling. Carbohydr. Polym. 2011, 83, 2005–2010. [Google Scholar] [CrossRef]
- Levdansky, V.A.; Kondracenko, A.S.; Levdansky, A.V.; Kuznetsov, B.N.; Djakovitch, L.; Pinel, C. Sulfation of microcrystalline cellulose with sulfamic acid in N,N-dimethylformamide and diglyme. J. Sib. Fed. Univ. Chem. 2014, 2, 162–169. [Google Scholar]
- Rantanen, J.; Dimic-Misic, K.; Kuusisto, J.; Maloney, T.C. The effect of micro and nanofibrillated cellulose water uptake on high filler content composite paper properties and furnish dewatering. Cellulose 2015, 22, 4003–4015. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cel-lulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Zhang, X.; You, T.-T.; Xu, F. Deep eutectic solvents (DESs) for cellulose dissolution: A mini-review. Cellulose 2019, 26, 205–213. [Google Scholar] [CrossRef]
- Ji, H.; Xiang, Z.; Qi, H.; Han, T.; Pranovich, A.; Song, T. Strategy towards one-step preparation of carboxylic cellulose nanocrystals and nanofibrils with high yield, carboxylation and highly stable dispersibility using innocuous citric acid. Green Chem. 2019, 21, 1956–1964. [Google Scholar] [CrossRef]
- Bisaria, V.S.; Ghose, T.K. Biodegradation of cellulosic materials: Substrates, microorganisms, enzymes and products. Enzym. Microb. Technol. 1981, 3, 90–104. [Google Scholar] [CrossRef]
- Zhang, C.-W.; Xia, S.-Q.; Ma, P.-S. Facile pretreatment of lignocellulosic biomass using deep eutectic solvents. Bioresour. Technol. 2016, 219, 1–5. [Google Scholar] [CrossRef]
- Hong, S.; Yuan, Y.; Li, P.P.; Zhang, K.T.; Lian, H.L.; Liimatainen, H. Enhancement of the nano-fibrillation of birch cellulose pretreated with natural deep eutectic solvent. Ind. Crop. Prod. 2020, 154, 112677. [Google Scholar] [CrossRef]
- Gu, J.; Catchmark, J.M.; Kaiser, E.Q.; Archibald, D.D. Quantification of cellulose nanowhiskers sulfate esterification levels. Carbohydr. Polym. 2013, 92, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Liou, R.-M.; Chen, S.-H.; Lai, C.-L.; Hung, M.-Y.; Huang, C.-H. Effect of ammonium groups of sulfonated polysulfone membrane on its pervaporation performance. Desalination 2011, 278, 91–97. [Google Scholar] [CrossRef]
- Zhang, K.; Brendler, E.; Geissler, A.; Fischer, S. Synthesis and spectroscopic analysis of cellulose sulfates with regulable total degrees of substitution and sulfation patterns via 13C NMR and FT Raman spectroscopy. Polymer 2011, 52, 26–32. [Google Scholar] [CrossRef]
- Gu, F.; Wang, W.X.; Cai, Z.S.; Xue, F.; Jin, Y.C.; Zhu, J.Y. Water retention value for characterizing fibrillation degree of cellu-losic fibers at micro and nanometer scales. Cellulose 2018, 25, 2861–2871. [Google Scholar] [CrossRef]
- Hamad, W.Y. Some microrheological aspects of wood pulp fibers subjected to fatigue loading. Cellulose 2020, 4, 51–56. [Google Scholar] [CrossRef]
- Lahtinen, P.; Liukkonen, S.; Pere, J.; Sneck, A.; Kangas, H. A Comparative Study of Fibrillated Fibers from Different Mechanical and Chemical Pulps. Bioresources 2014, 9, 2115–2127. [Google Scholar] [CrossRef]
- Tian, D.; Guo, Y.; Hu, J.; Yang, G.; Zhang, J.; Luo, L.; Xiao, Y.; Deng, S.; Deng, O.; Zhou, W.; et al. Acidic deep eutectic solvents pretreatment for selective lignocellulosic biomass fractionation with enhanced cellulose reactivity. Int. J. Biol. Macromol. 2019, 142, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, A.; Ravishankar, K.; Dhamodharan, R. Preparation of nanofibrillated cellulose and nanocrystalline cellulose from surgical cotton and cellulose pulp in hot-glycerol medium. Cellulose 2019, 26, 3127–3141. [Google Scholar] [CrossRef]
- Dupont, D.; Renders, E.; Raiguel, S.; Binnemans, K. New metal extractants and super-acidic ionic liquids derived from sulfamic acid. Chem. Commun. 2016, 52, 7032–7035. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Liu, W.; Zhang, L.; Ouyang, H.; Hou, Q.; Fan, K.; Li, J.; Liu, P.; Liu, X. Fabricating Cellulose Nanofibril from Licorice Residues and Its Cellulose Composite Incorporated with Natural Nanoparticles. Carbohydr. Polym. 2020, 229, 115464–115494. [Google Scholar] [CrossRef]
- Sharma, A.; Mandal, S.; Goswami, S. Dispersibility and stability studies of cellulose nanofibers: Implications for nanocompo-site preparation. J. Polym. Environ. 2020, 29, 1516–1525. [Google Scholar] [CrossRef]
- Campano, C.; Balea, A.; Blanco, Á.; Negro, C. A reproducible method to characterize the bulk morphology of cellulose nanocrystals and nanofibers by transmission electron microscopy. Cellulose 2020, 27, 4871–4887. [Google Scholar] [CrossRef]
- Pai, A.R.; Binumol, T.; Gopakumar, D.A.; Pasquini, D.; Thomas, S. Ultra-fast heat dissipating aerogels derived from polyaniline anchored cellulose nanofibers as sustainable microwave absorbers. Carbohydr. Polym. 2020, 246, 116663. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, B.; Xia, Q.; Meng, J.; Chen, W.; Liu, S.; Wang, Q.; Liu, Y.; Li, J.; Yu, H. Efficient Cleavage of Strong Hydrogen Bonds in Cotton by Deep Eutectic Solvents and Facile Fabrication of Cellulose Nanocrystals in High Yields. ACS Sustain. Chem. Eng. 2017, 5, 7623–7631. [Google Scholar] [CrossRef]
- Zhang, K.T.; Sun, P.P.; Liu, H.; Shang, S.B.; Song, J.; Wang, D. Extraction and comparison of carboxylated cellulose nano-crystals from bleached sugarcane bagasse pulp using two different oxidation methods. Carbohydr. Polym. 2016, 138, 237–243. [Google Scholar] [CrossRef]
- Li, P.P.; Sirviö, J.A.; Hong, S.; Ämmälä, A.; Liimatainen, H. Preparation of flame-retardant lignin-containing wood nano-fibers using a high-consistency mechano-chemical pretreatment. Chem. Eng. J. 2019, 375, 122050. [Google Scholar] [CrossRef]
- Niu, X.; Liu, Y.; Fang, G.; Huang, C.; Rojas, O.J.; Pan, H. Highly Transparent, Strong, and Flexible Films with Modified Cellulose Nanofiber Bearing UV Shielding Property. Biomacromolecules 2018, 19, 4565–4575. [Google Scholar] [CrossRef]
- Chee, C.Y.; Ashiqur, R.; Yong, C.K.; Liana, S.N.; Chuah, C.H. Preparation and characterization of polyvinyl alcohol-based composite reinforced with nanocellulose and nanosilica. Bioresources 2015, 10, 211–216. [Google Scholar]
- Xiao, S.; Gao, R.; Gao, L.; Li, J. Poly(vinyl alcohol) films reinforced with nanofibrillated cellulose (NFC) isolated from corn husk by high intensity ultrasonication. Carbohydr. Polym. 2016, 136, 1027–1034. [Google Scholar] [CrossRef] [PubMed]









| pH | Viscosity (mPa·s) | Melting Point (°C) |
|---|---|---|
| 2.36 ± 0.03 | 1389 ± 2 | −63.6 ± 0.1 |
| Sample | Sample | Sulfamic Acid:Cellulose Mass Ratio | Time (h) |
|---|---|---|---|
| Pulp-6-1 | CNF-6-1 | 1:6 | 1 |
| Pulp-6-1.5 | CNF-6-1.5 | 1:6 | 1.5 |
| Pulp-9-1 | CNF-9-1 | 1:9 | 1 |
| Pulp-9-1.5 | CNF-9-1.5 | 1:9 | 1.5 |
| Pulp-12-1 | CNF-12-1 | 1:12 | 1 |
| Pulp-12-1.5 | CNF-12-1.5 | 1:12 | 1.5 |
| Sample | Yield (%) | Width (µm) | DP | S (mmol/g) | 1 DS | Sample | ZP (mV) | CrI (%) |
|---|---|---|---|---|---|---|---|---|
| Original cellulose pulp | - | 13.4 | 1012 | 0.00 | 0.00 | CNF | −18.3 | 64.0 |
| Pulp-6-1 | 95.2 | 15.5 | 638 | 0.18 | 0.01 | CNF-6-1 | −29.4 | 61.7 |
| Pulp-6-1.5 | 86.5 | 15.6 | 517 | 0.50 | 0.09 | CNF-6-1.5 | −30.2 | 60.2 |
| Pulp-9-1 | 94.9 | 15.8 | 517 | 0.20 | 0.03 | CNF-9-1 | −31.0 | 57.6 |
| Pulp-9-1.5 | 84.5 | 17.3 | 460 | 0.53 | 0.09 | CNF-9-1.5 | −31.8 | 54.5 |
| Pulp-12-1 | 92.2 | 16.9 | 513 | 0.28 | 0.05 | CNF-12-1 | −31.3 | 54.8 |
| Pulp-12-1.5 | 80.9 | 17.4 | 412 | 0.70 | 0.12 | CNF-12-1.5 | −35.2 | 53.3 |
| Sample | Young’s Modulus (MPa) | Maximum Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| PVA | 221 ± 74 | 11 ± 3 | 8.9 ± 0.4 |
| PVA-CNF | 444 ± 68 | 19 ± 2 | 8.4 ± 0.2 |
| PVA-CNF-6-1 | 569 ± 59 | 23 ± 2 | 7.5 ± 0.2 |
| PVA-CNF-6-1.5 | 602 ± 81 | 31 ± 4 | 6.2 ± 0.1 |
| PVA-CNF-9-1 | 576 ± 93 | 26 ± 3 | 7.2 ± 0.3 |
| PVA-CNF-9-1.5 | 1493 ± 101 | 40 ± 5 | 5.8 ± 0.1 |
| PVA-CNF-12-1 | 584 ± 76 | 27 ± 7 | 6.8 ± 0.8 |
| PVA-CNF-12-1.5 | 1529 ± 128 | 44 ± 3 | 5.5 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Xue, Y.; He, M.; Yan, J.; Lucia, L.A.; Chen, J.; Yu, J.; Yang, G. Facile Preparation and Characteristic Analysis of Sulfated Cellulose Nanofibril via the Pretreatment of Sulfamic Acid-Glycerol Based Deep Eutectic Solvents. Nanomaterials 2021, 11, 2778. https://doi.org/10.3390/nano11112778
Li W, Xue Y, He M, Yan J, Lucia LA, Chen J, Yu J, Yang G. Facile Preparation and Characteristic Analysis of Sulfated Cellulose Nanofibril via the Pretreatment of Sulfamic Acid-Glycerol Based Deep Eutectic Solvents. Nanomaterials. 2021; 11(11):2778. https://doi.org/10.3390/nano11112778
Chicago/Turabian StyleLi, Weidong, Yu Xue, Ming He, Jiaqiang Yan, Lucian A. Lucia, Jiachuan Chen, Jinghua Yu, and Guihua Yang. 2021. "Facile Preparation and Characteristic Analysis of Sulfated Cellulose Nanofibril via the Pretreatment of Sulfamic Acid-Glycerol Based Deep Eutectic Solvents" Nanomaterials 11, no. 11: 2778. https://doi.org/10.3390/nano11112778