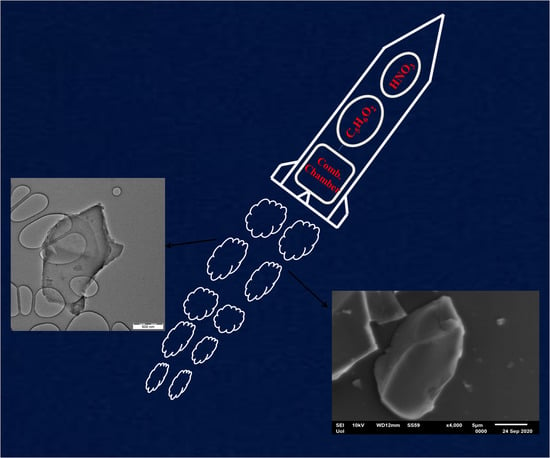
Nanocarbon from Rocket Fuel Waste: The Case of Furfuryl Alcohol-Fuming Nitric Acid Hypergolic Pair
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Iroegbu, A.O.; Hlangothi, S.P. Furfuryl alcohol a versatile, eco-sustainable compound in perspective. Chem. Afr. 2019, 2, 223–239. [Google Scholar] [CrossRef] [Green Version]
- Munjal, N.L. Ignition catalysts for furfuryl alcohol—Red fuming nitric acid bipropellant. AIAA J. 1970, 8, 980–981. [Google Scholar] [CrossRef]
- Kulkarni, S.G.; Bagalkote, V.S.; Patil, S.S.; Kumar, U.P.; Kumar, V.A. Theoretical evaluation and experimental validation of performance parameters of new hypergolic liquid fuel blends with red fuming nitric acid as oxidizer. Propellants Explos. Pyrotech. 2009, 34, 520–525. [Google Scholar] [CrossRef]
- Kulkarni, S.; Bagalkote, V. Studies on pre-ignition reactions of hydrocarbon-based rocket fuels hypergolic with red fuming nitric acid as oxidizer. J. Energet. Mater. 2010, 28, 173–188. [Google Scholar] [CrossRef]
- Bhosale, M.V.K.; Kulkarni, S.G.; Kulkarni, P.S. Ionic liquid and biofuel blend: A low–cost and high performance hypergolic fuel for propulsion application. ChemistrySelect 2016, 1, 1921–1925. [Google Scholar] [CrossRef]
- Kyotani, T.; Nagai, T.; Inoue, S.; Tomita, A. Formation of new type of porous carbon by carbonization in zeolite nanochannels. Chem. Mater. 1997, 9, 609–615. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Zhang, L. Highly dispersible molecular sieve carbon nanoparticles. Chem. Mater. 2004, 16, 4205–4207. [Google Scholar] [CrossRef]
- Janus, P.; Janus, R.; Kuśtrowski, P.; Jarczewski, S.; Wach, A.; Silvestre-Albero, A.M.; Rodríguez-Reinoso, F. Chemically activated poly(furfuryl alcohol)-derived CMK-3 carbon catalysts for the oxidative dehydrogenation of ethylbenzene. Catal. Today 2014, 235, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Lorenc-Grabowska, E.; Rutkowski, P. Tailoring mesoporosity of poly(furfuryl alcohol)-based activated carbons and their ability to adsorb organic compounds from water. J. Mater. Cycles Waste Manag. 2018, 20, 1638–1647. [Google Scholar] [CrossRef] [Green Version]
- Węgrzyniak, A.; Jarczewski, S.; Kuśtrowski, P.; Michorczyk, P. Influence of carbon precursor on porosity, surface composition and catalytic behaviour of CMK-3 in oxidative dehydrogenation of propane to propene. J. Porous Mater. 2018, 25, 687–696. [Google Scholar] [CrossRef] [Green Version]
- Arnaiz, M.; Nair, V.; Mitra, S.; Ajuria, J. Furfuryl alcohol derived high-end carbons for ultrafast dual carbon lithium ion capacitors. Electrochim. Acta 2019, 304, 437–446. [Google Scholar] [CrossRef]
- Singh, J.; Basu, S.; Bhunia, H. Furfuryl alcohol-derived carbon monoliths for CO2 capture: Adsorption isotherm and kinetic study. IOP Conf. Ser. Mater. Sci. Eng. 2019, 625, 012014. [Google Scholar] [CrossRef]
- Janus, P.; Janus, R.; Dudek, B.; Drozdek, M.; Silvestre-Albero, A.; Rodríguez-Reinoso, F.; Kuśtrowski, P. On mechanism of formation of SBA-15/furfuryl alcohol-derived mesoporous carbon replicas and its relationship with catalytic activity in oxidative dehydrogenation of ethylbenzene. Microporous Mesoporous Mater. 2020, 299, 110118. [Google Scholar] [CrossRef]
- Baikousi, M.; Chalmpes, N.; Spyrou, K.; Bourlinos, A.B.; Avgeropoulos, A.; Gournis, D.; Karakassides, M.A. Direct production of carbon nanosheets by self-ignition of pyrophoric lithium dialkylamides in air. Mater. Lett. 2019, 254, 58–61. [Google Scholar] [CrossRef]
- Chalmpes, N.; Spyrou, K.; Bourlinos, A.B.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Synthesis of highly crystalline graphite from spontaneous ignition of in situ derived acetylene and chlorine at ambient conditions. Molecules 2020, 25, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalmpes, N.; Asimakopoulos, G.; Spyrou, K.; Vasilopoulos, K.C.; Bourlinos, A.B.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Functional carbon materials derived through hypergolic reactions at ambient conditions. Nanomaterials 2020, 10, 566. [Google Scholar] [CrossRef] [Green Version]
- Chalmpes, N.; Spyrou, K.; Vasilopoulos, K.C.; Bourlinos, A.B.; Moschovas, D.; Avgeropoulos, A.; Gioti, C.; Karakassides, M.A.; Gournis, D. Hypergolics in carbon nanomaterials synthesis: New paradigms and perspectives. Molecules 2020, 25, 2207. [Google Scholar] [CrossRef]
- Chalmpes, N.; Tantis, I.; Bakandritsos, A.; Bourlinos, A.B.; Karakassides, M.A.; Gournis, D. Rapid carbon formation from spontaneous reaction of ferrocene and liquid bromine at ambient conditions. Nanomaterials 2020, 10, 1564. [Google Scholar] [CrossRef]
- Chalmpes, N.; Bourlinos, A.B.; Šedajová, V.; Kupka, V.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Hypergolic materials synthesis through reaction of fuming nitric acid with certain cyclopentadienyl compounds. C—J. Carbon Res. 2020, 6, 61. [Google Scholar] [CrossRef]
- Choura, M.; Belgacem, N.M.; Gandini, A. Acid-Catalyzed Polycondensation of furfuryl alcohol: Mechanisms of chromophore formation and cross-linking. Macromolecules 1996, 29, 3839–3850. [Google Scholar] [CrossRef]
- Guigo, N.; Mija, A.; Zavaglia, R.; Vincent, L.; Sbirrazzuoli, N. New insights on the thermal degradation pathways of neat poly(furfuryl alcohol) and poly(furfuryl alcohol)/SiO2 hybrid materials. Polym. Degrad. Stab. 2009, 94, 908–913. [Google Scholar] [CrossRef]
- Ahmad, E.E.M.; Luyt, A.S.; Djoković, V. Thermal and dynamic mechanical properties of bio-based poly(furfuryl alcohol)/sisal whiskers nanocomposites. Polym. Bull. 2013, 70, 1265–1276. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Z.; Huang, Y.; Xue, R.; Huang, X.; Chen, L. Characterizations of crystalline structure and electrical properties of pyrolyzed polyfurfuryl alcohol. J. Appl. Phys. 1997, 82, 5705–5710. [Google Scholar] [CrossRef]
- Almeida Filho, C.D.; Zarbin, A.J.G. Porous carbon obtained by the pyrolysis of TiO2/poly(furfuryl alcohol) nanocomposite: Preparation, characterization and utilization for adsorption of reactive dyes from aqueous solution. J. Braz. Chem. Soc. 2006, 17, 1151–1157. [Google Scholar] [CrossRef] [Green Version]
- Tsirka, K.; Katsiki, A.; Chalmpes, N.; Gournis, D.; Paipetis, A.S. Mapping of graphene oxide and single layer graphene flakes—defects annealing and healing. Front. Mater. 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Rommozzi, E.; Zannotti, M.; Giovannetti, R.; D’Amato, C.A.; Ferraro, S.; Minicucci, M.; Gunnella, R.; Di Cicco, A. Reduced graphene oxide/TiO2 nanocomposite: From synthesis to characterization for efficient visible light photocatalytic applications. Catalysts 2018, 8, 598. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Hu, N.; Yang, C.; Wei, H.; Yang, Z.; Wang, Y.; Wei, L.; Zhao, J.; Xu, Z.J.; Zhang, Y. Free-standing functional graphene reinforced carbon films with excellent mechanical properties and superhydrophobic characteristic. Compos. Part A Appl. Sci. Manuf. 2015, 74, 96–106. [Google Scholar] [CrossRef]
- D’Arsié, L.; Esconjauregui, S.; Weatherup, R.S.; Wu, X.; Arter, W.E.; Sugime, H.; Cepek, C.; Robertson, J. Stable, efficient p-type doping of graphene by nitric acid. RSC Adv. 2016, 6, 113185–113192. [Google Scholar] [CrossRef] [Green Version]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, L.; Wang, K.; Miao, L.; Lan, Q.; Jiang, K.; Lu, H.; Li, M.; Li, Y.; Shen, B.; et al. Analysis of oxidation degree of graphite oxide and chemical structure of corresponding reduced graphite oxide by selecting different-sized original graphite. RSC Adv. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Kumar, B.; Asadi, M.; Pisasale, D.; Sinha-Ray, S.; Rosen, B.A.; Haasch, R.; Abiade, J.; Yarin, A.L.; Salehi-Khojin, A. Renewable and metal-free carbon nanofibre catalysts for carbon dioxide reduction. Nat. Commun. 2013, 4, 2819. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Safarova, K.; Siskova, K.; Zbořil, R. The production of chemically converted graphenes from graphite fluoride. Carbon 2012, 50, 1425–1428. [Google Scholar] [CrossRef]
- Munjal, N.L.; Parvatiyar, M.G. Ignition of hybrid rocket fuels with fuming nitric acid as oxidant. J. Spacecr. Rocket. 1974, 11, 428–430. [Google Scholar] [CrossRef]
- Durgapal, U.C.; Dutta, P.K.; Pant, G.C.; Ingalgaonkar, M.B.; Oka, V.Y.; Umap, B.B. Studies on hypergolicity of several liquid fuels with fuming nitric acids as oxidizers. Propellants Explos. Pyrotech. 1987, 12, 149–153. [Google Scholar] [CrossRef]
- Hollingshead, J.; Litzinger, M.; Kiaoulias, D.; Eckenrode, L.; Moore, J.D.; Risha, G.A.; Yetter, R.A. Combustion of a TMEDA/WFNA hypergolic in a bipropellant rocket engine. In Proceedings of the AIAA Propulsion and Energy 2019 Forum, Indianapolis, IN, USA, 19–22 August 2019. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalmpes, N.; Bourlinos, A.B.; Talande, S.; Bakandritsos, A.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Nanocarbon from Rocket Fuel Waste: The Case of Furfuryl Alcohol-Fuming Nitric Acid Hypergolic Pair. Nanomaterials 2021, 11, 1. https://doi.org/10.3390/nano11010001
Chalmpes N, Bourlinos AB, Talande S, Bakandritsos A, Moschovas D, Avgeropoulos A, Karakassides MA, Gournis D. Nanocarbon from Rocket Fuel Waste: The Case of Furfuryl Alcohol-Fuming Nitric Acid Hypergolic Pair. Nanomaterials. 2021; 11(1):1. https://doi.org/10.3390/nano11010001
Chicago/Turabian StyleChalmpes, Nikolaos, Athanasios B. Bourlinos, Smita Talande, Aristides Bakandritsos, Dimitrios Moschovas, Apostolos Avgeropoulos, Michael A. Karakassides, and Dimitrios Gournis. 2021. "Nanocarbon from Rocket Fuel Waste: The Case of Furfuryl Alcohol-Fuming Nitric Acid Hypergolic Pair" Nanomaterials 11, no. 1: 1. https://doi.org/10.3390/nano11010001